Epidemiology and natural history of central venous access device use and infusion pump function in the NO16966 trial
- PMID: 24548866
- PMCID: PMC3960626
- DOI: 10.1038/bjc.2014.74
Epidemiology and natural history of central venous access device use and infusion pump function in the NO16966 trial
Abstract
Background: Central venous access devices in fluoropyrimidine therapy are associated with complications; however, reliable data are lacking regarding their natural history, associated complications and infusion pump performance in patients with metastatic colorectal cancer.
Methods: We assessed device placement, use during treatment, associated clinical outcomes and infusion pump performance in the NO16966 trial.
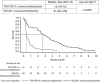
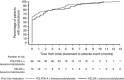
Results: Device replacement was more common with FOLFOX-4 (5-fluorouracil (5-FU)+oxaliplatin) than XELOX (capecitabine+oxaliplatin) (14.1% vs 5.1%). Baseline device-associated events and post-baseline removal-/placement-related events occurred more frequently with FOLFOX-4 than XELOX (11.5% vs 2.4% and 8.5% vs 2.1%). Pump malfunctions, primarily infusion accelerations in 16% of patients, occurred within 1.6-4.3% of cycles. Fluoropyrimidine-associated grade 3/4 toxicity was increased in FOLFOX-4-treated patients experiencing a malfunction compared with those who did not (97 out of 155 vs 452 out of 825 patients), predominantly with increased grade 3/4 neutropenia (53.5% vs 39.8%). Febrile neutropenia rates were comparable between patient cohorts±malfunction. Efficacy outcomes were similar in patient cohorts±malfunction.
Conclusions: Central venous access device removal or replacement was common and more frequent in patients receiving FOLFOX-4. Pump malfunctions were also common and were associated with increased rates of grade 3/4 haematological adverse events. Oral fluoropyrimidine-based regimens may be preferable to infusional 5-FU based on these findings.
Figures



References
-
- Araújo C, Silva JP, Antunes P, Fernandes JM, Dias C, Pereira H, Dias T, Fougo JL. A comparative study between two central veins for the introduction of totally implantable venous access devices in 1201 cancer patients. Eur J Surg Oncol. 2008;34 (2:222–226. - PubMed
-
- Barbetakis N, Asteriou C, Kleontas A, Tsilikas C. Totally implantable central venous access ports. Analysis of 700 cases. J Surg Oncol. 2011;104 (6:654–656. - PubMed
-
- Beckers MM, Ruven HJ, Seldenrijk CA, Prins MH, Biesma DH. Risk of thrombosis and infections of central venous catheters and totally implanted access ports in patients treated for cancer. Thromb Res. 2010;125 (4:318–321. - PubMed
-
- Biffi R, Orsi F, Pozzi S, Pace U, Bonomo G, Monfardini L, Della Vigna P, Rotmensz N, Radice D, Zampino MG, Fazio N, de Braud F, Andreoni B, Goldhirsch A. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20 (5:935–940. - PubMed
-
- Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang T-S, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26 (12:2006–2012. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

