Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency
- PMID: 24549041
- PMCID: PMC4049311
- DOI: 10.1093/hmg/ddu069
Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency
Abstract
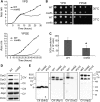
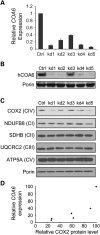
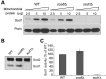
Mitochondrial respiratory chain biogenesis is orchestrated by hundreds of assembly factors, many of which are yet to be discovered. Using an integrative approach based on clues from evolutionary history, protein localization and human genetics, we have identified a conserved mitochondrial protein, C1orf31/COA6, and shown its requirement for respiratory complex IV biogenesis in yeast, zebrafish and human cells. A recent next-generation sequencing study reported potential pathogenic mutations within the evolutionarily conserved Cx₉CxnCx₁₀C motif of COA6, implicating it in mitochondrial disease biology. Using yeast coa6Δ cells, we show that conserved residues in the motif, including the residue mutated in a patient with mitochondrial disease, are essential for COA6 function, thus confirming the pathogenicity of the patient mutation. Furthermore, we show that zebrafish embryos with zfcoa6 knockdown display reduced heart rate and cardiac developmental defects, recapitulating the observed pathology in the human mitochondrial disease patient who died of neonatal hypertrophic cardiomyopathy. The specific requirement of Coa6 for respiratory complex IV biogenesis, its intramitochondrial localization and the presence of the Cx₉CxnCx₁₀C motif suggested a role in mitochondrial copper metabolism. In support of this, we show that exogenous copper supplementation completely rescues respiratory and complex IV assembly defects in yeast coa6Δ cells. Taken together, our results establish an evolutionarily conserved role of Coa6 in complex IV assembly and support a causal role of the COA6 mutation in the human mitochondrial disease patient.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
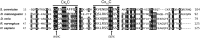




References
-
- Skladal D., Halliday J., Thorburn D.R. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126:1905–1912. - PubMed
-
- DiMauro S., Schon E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 2003;348:2656–2668. - PubMed
-
- Fernández-Vizarra E., Tiranti V., Zeviani M. Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim. Biophys. Acta. 2009;1793:200–211. - PubMed
-
- Vafai S.B., Mootha V.K. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

