14-3-3 and aggresome formation: implications in neurodegenerative diseases
- PMID: 24549097
- PMCID: PMC4189886
- DOI: 10.4161/pri.28123
14-3-3 and aggresome formation: implications in neurodegenerative diseases
Abstract
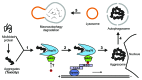
Protein misfolding and aggregation underlie the pathogenesis of many neurodegenerative diseases. In addition to chaperone-mediated refolding and proteasomal degradation, the aggresome-macroautophagy pathway has emerged as another defense mechanism for sequestration and clearance of toxic protein aggregates in cells. Previously, the 14-3-3 proteins were shown to be indispensable for the formation of aggresomes induced by mutant huntingtin proteins. In a recent study, we have determined that 14-3-3 functions as a molecular adaptor to recruit chaperone-associated misfolded proteins to dynein motors for transport to aggresomes. This molecular complex involves a dimeric binding of 14-3-3 to both the dynein-intermediate chain (DIC) and an Hsp70 co-chaperone Bcl-2-associated athanogene 3 (BAG3). As 14-3-3 has been implicated in various neurodegenerative diseases, our findings may provide mechanistic insights into its role in managing misfolded protein stress during the process of neurodegeneration.
Keywords: 14-3-3; aggresomes; chaperones; inclusion bodies; neurodegeneration; protein aggregation; protein misfolding.
Figures
Comment on
- Xu Z, Graham K, Foote M, Liang F, Rizkallah R, Hurt M, Wang Y, Wu Y, Zhou Y. 14-3-3 protein targets chaperone-associated misfolded proteins to aggresomes. J Cell Sci. 2013;126:4173–86. doi: 10.1242/jcs.126102. doi: 10.1242/jcs.126102
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
