Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically
- PMID: 24549183
- PMCID: PMC4013765
- DOI: 10.1038/jcbfm.2014.28
Systematic reviews and meta-analysis of preclinical studies: why perform them and how to appraise them critically
Abstract
The use of systematic review and meta-analysis of preclinical studies has become more common, including those of studies describing the modeling of cerebrovascular diseases. Empirical evidence suggests that too many preclinical experiments lack methodological rigor, and this leads to inflated treatment effects. The aim of this review is to describe the concepts of systematic review and meta-analysis and consider how these tools may be used to provide empirical evidence to spur the field to improve the rigor of the conduct and reporting of preclinical research akin to their use in improving the conduct and reporting of randomized controlled trials in clinical research. As with other research domains, systematic reviews are subject to bias. Therefore, we have also suggested guidance for their conduct, reporting, and critical appraisal.
Figures
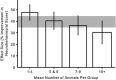
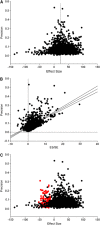
References
-
- Glass G.Meta-analysis at 25 http://www.gvglassinfo/papers/meta25.html2013
-
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. - PubMed
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical-evidence of bias—dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. - PubMed
-
- Chalmers TC, Celano P, Sacks HS, Smith H. Bias in treatment assignment in controlled clinical trials. N Engl J Med. 1983;309:1358–1361. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

