Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells
- PMID: 24549276
- PMCID: PMC4138288
- DOI: 10.1038/mi.2014.10
Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells
Abstract
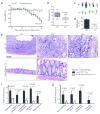
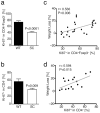
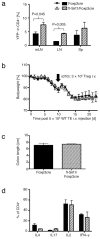
Induced Forkhead box P3-positive (Foxp3(+)) T-regulatory cells (iTregs) are essential to gastrointestinal immune homeostasis, and loss of the ability to develop iTregs may lead to autoimmune colitis. We previously showed a role for sirtuin-1 (Sirt1) in control of Treg function and hypothesized that targeting of Sirt1 might enhance iTreg development and thereby represent a potential therapy for inflammatory bowel disease (IBD). We adoptively transferred CD4(+)CD25(-)Foxp3(-) T effector (TE) cells from wild-type (WT) (C57BL/6) or fl-Sirt1/CD4cre mice into B6/Rag1(-/-) mice and monitored the mice until they lost 10-15% of their weight. Adoptive transfer of TE cells lacking Sirt1 to B6/Rag1(-/-) mice resulted in a 2.8-fold increase in iTreg formation compared with mice receiving WT TE cells and correlated with attenuated colitis and reduced weight loss (1.04±1.4% vs. 13.97±2.2%, respectively, P<0.001). In a second model of IBD, we used pharmacologic Sirt1 targeting of mice receiving multiple cycles of dextran sodium sulfate (DSS) in their drinking water, alternated with fresh water. Likewise, WT mice receiving cyclic DSS and a Sirt1 inhibitor, EX-527, had reduced weight loss (5.8±5.9% vs. 13.2±6.9%, respectively, P=0.03) and increased iTreg formation compared with controls. Sirt1 appears a promising target for pharmacologic therapy of IBD as a result of promoting iTreg development.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
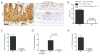






References
-
- Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307(5717):1920–1925. - PubMed
-
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annual review of immunology. 2009;27:313–338. - PubMed
-
- Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–635. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

