Structural insight into the dimerization of human protein disulfide isomerase
- PMID: 24549644
- PMCID: PMC4005713
- DOI: 10.1002/pro.2444
Structural insight into the dimerization of human protein disulfide isomerase
Abstract
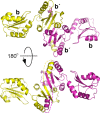
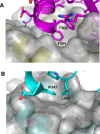
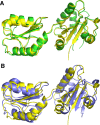
Protein disulfide isomerases (PDIs) are responsible for catalyzing the proper oxidation and isomerization of disulfide bonds of newly synthesized proteins in the endoplasmic reticulum (ER). Here, it is shown that human PDI (PDIA1) dimerizes in vivo and proposed that the dimerization of PDI has physiological relevance by autoregulating its activity. The crystal structure of the dimeric form of noncatalytic bb' domains of human PDIA1 determined to 2.3 Å resolution revealed that the formation of dimers occludes the substrate binding site and may function as a mechanism to regulate PDI activity in the ER.
Keywords: crystal structure; dimerization; endoplasmic reticulum; protein disulfide isomerase; thioredoxin-like domain.
© 2014 The Protein Society.
Figures



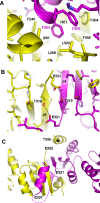
References
-
- Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. - PubMed
-
- Karala AR, Lappi AK, Saaranen MJ, Ruddock LW. Efficient peroxide-mediated oxidative refolding of a protein at physiological pH and implications for oxidative folding in the endoplasmic reticulum. Antiox Redox Signal. 2009;11:963–970. - PubMed
-
- Benham AM. The protein disulfide isomerase family: key players in health and disease. Antiox Redox Signal. 2012;16:781–789. - PubMed
-
- Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H. The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell. 2006;124:61–73. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

