Identification of a novel m.9588G > a missense mutation in the mitochondrial COIII gene in asthenozoospermic Tunisian infertile men
- PMID: 24550096
- PMCID: PMC4016372
- DOI: 10.1007/s10815-014-0187-2
Identification of a novel m.9588G > a missense mutation in the mitochondrial COIII gene in asthenozoospermic Tunisian infertile men
Abstract
Purpose: Infertility affects 10-15 % of the population, of which, approximately 40 % is due to male etiology consisting primarily of low sperm count (oligozoospermia) and/or abnormal sperm motility (asthenozoospermia). It has been demonstrated that mtDNA base substitutions can greatly influence semen quality.
Methods: In the present study we performed a systematic sequence analysis of the mitochondrial cytochrome oxidase III (COIII) gene in 31 asthenozoospermic infertile men in comparaison to normozoospermic infertile men (n=33) and fertile men (n=150) from Tunisian population.
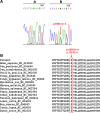
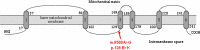
Results: A novel m.9588G>A mutation was found in the mtDNA sperm's in all asthenozoospermic patients and was absent in the normozoospermic and in fertile men. The m.9588G>A mutation substitutes a highly conserved Glutamate at position 128 to Lysine. In addition, PolyPhen-2 analysis predicted that this variant is "probably damaging".
Figures

References
-
- Følgero T, Bertheussen K, Lindal S, Torbergsen T, Oian P. Mitochondrial disease and reduced sperm motility. Hum Reprod. 1993;8:1863–1868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

