Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells
- PMID: 24550116
- PMCID: PMC3929409
- DOI: 10.1242/dev.103333
Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells
Abstract
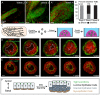
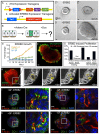
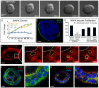
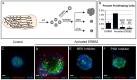
Mammary ducts are elongated during development by stratified epithelial structures, known as terminal end buds (TEBs). TEBs exhibit reduced apicobasal polarity and extensive proliferation. A major unanswered question concerns the mechanism by which the simple ductal epithelium stratifies during TEB formation. We sought to elucidate this mechanism using real-time imaging of growth factor-induced stratification in 3D cultures of mouse primary epithelial organoids. We hypothesized that stratification could result from vertical divisions in either the apically positioned luminal epithelial cells or the basally positioned myoepithelial cells. Stratification initiated exclusively from vertical apical cell divisions, both in 3D culture and in vivo. During vertical apical divisions, only the mother cell retained tight junctions and segregated apical membranes. Vertical daughter cells initiated an unpolarized cell population located between the luminal and myoepithelial cells, similar to the unpolarized body cells in the TEB. As stratification and loss of apicobasal polarity are early hallmarks of cancer, we next determined the cellular mechanism of oncogenic stratification. Expression of activated ERBB2 induced neoplastic stratification through analogous vertical divisions of apically positioned luminal epithelial cells. However, ERBB2-induced stratification was accompanied by tissue overgrowth and acute loss of both tight junctions and apical polarity. Expression of phosphomimetic MEK (MEK1DD), a major ERBB2 effector, also induced stratification through vertical apical cell divisions. However, MEK1DD-expressing organoids exhibited normal levels of growth and retained apicobasal polarity. We conclude that both normal and neoplastic stratification are accomplished through receptor tyrosine kinase signaling dependent vertical cell divisions within the luminal epithelial cell layer.
Keywords: Apicobasal polarity; Breast cancer; Epithelial development; Mammary gland; Mouse.
Figures


Similar articles
-
Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium.J Cell Sci. 2012 Jun 1;125(Pt 11):2638-54. doi: 10.1242/jcs.096875. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344263 Free PMC article.
-
p63 is a prosurvival factor in the adult mammary gland during post-lactational involution, affecting PI-MECs and ErbB2 tumorigenesis.Cell Death Differ. 2014 Apr;21(4):645-54. doi: 10.1038/cdd.2013.199. Epub 2014 Jan 17. Cell Death Differ. 2014. PMID: 24440910 Free PMC article.
-
Single cell transcriptome atlas of mouse mammary epithelial cells across development.Breast Cancer Res. 2021 Jun 29;23(1):69. doi: 10.1186/s13058-021-01445-4. Breast Cancer Res. 2021. PMID: 34187545 Free PMC article.
-
Myoepithelial cells in the control of mammary development and tumorigenesis: data from genetically modified mice.J Mammary Gland Biol Neoplasia. 2005 Jul;10(3):211-9. doi: 10.1007/s10911-005-9582-8. J Mammary Gland Biol Neoplasia. 2005. PMID: 16807801 Review.
-
Sequestration and segregation of receptor kinases in epithelial cells: implications for ErbB2 oncogenesis.Sci STKE. 2007 Apr 10;2007(381):re3. doi: 10.1126/stke.3812007re3. Sci STKE. 2007. PMID: 17426346 Review.
Cited by
-
A junction-dependent mechanism drives murine mammary cell intercalation for ductal elongation.Dev Cell. 2023 Jul 10;58(13):1126-1138.e4. doi: 10.1016/j.devcel.2023.04.009. Epub 2023 May 3. Dev Cell. 2023. PMID: 37141887 Free PMC article.
-
Sporadic activation of an oxidative stress-dependent NRF2-p53 signaling network in breast epithelial spheroids and premalignancies.Sci Signal. 2020 Apr 14;13(627):eaba4200. doi: 10.1126/scisignal.aba4200. Sci Signal. 2020. PMID: 32291314 Free PMC article.
-
Erbin interacts with NHERF1 and Ezrin to stabilize a membrane ErbB2 signaling complex in HER2-positive breast cancer.Breast Cancer Res. 2025 May 19;27(1):85. doi: 10.1186/s13058-025-02025-6. Breast Cancer Res. 2025. PMID: 40390040 Free PMC article.
-
Collective cell migration is spatiotemporally regulated during mammary epithelial bifurcation.J Cell Sci. 2023 Jan 1;136(1):jcs259275. doi: 10.1242/jcs.259275. Epub 2023 Jan 5. J Cell Sci. 2023. PMID: 36602106 Free PMC article.
-
3D culture assays of murine mammary branching morphogenesis and epithelial invasion.Methods Mol Biol. 2015;1189:135-62. doi: 10.1007/978-1-4939-1164-6_10. Methods Mol Biol. 2015. PMID: 25245692 Free PMC article.
References
-
- Aranda V., Haire T., Nolan M. E., Calarco J. P., Rozenberg A. Z., Fawcett J. P., Pawson T., Muthuswamy S. K. (2006). Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat. Cell Biol. 8, 1235–1245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

