Role of vascular oxidative stress in obesity and metabolic syndrome
- PMID: 24550188
- PMCID: PMC4066332
- DOI: 10.2337/db13-0719
Role of vascular oxidative stress in obesity and metabolic syndrome
Abstract
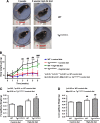
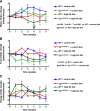
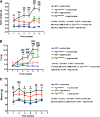
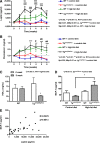
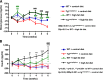
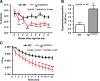
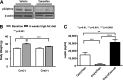
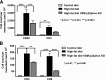
Obesity is associated with vascular diseases that are often attributed to vascular oxidative stress. We tested the hypothesis that vascular oxidative stress could induce obesity. We previously developed mice that overexpress p22phox in vascular smooth muscle, tg(sm/p22phox), which have increased vascular ROS production. At baseline, tg(sm/p22phox) mice have a modest increase in body weight. With high-fat feeding, tg(sm/p22phox) mice developed exaggerated obesity and increased fat mass. Body weight increased from 32.16 ± 2.34 g to 43.03 ± 1.44 g in tg(sm/p22phox) mice (vs. 30.81 ± 0.71 g to 37.89 ± 1.16 g in the WT mice). This was associated with development of glucose intolerance, reduced HDL cholesterol, and increased levels of leptin and MCP-1. Tg(sm/p22phox) mice displayed impaired spontaneous activity and increased mitochondrial ROS production and mitochondrial dysfunction in skeletal muscle. In mice with vascular smooth muscle-targeted deletion of p22phox (p22phox(loxp/loxp)/tg(smmhc/cre) mice), high-fat feeding did not induce weight gain or leptin resistance. These mice also had reduced T-cell infiltration of perivascular fat. In conclusion, these data indicate that vascular oxidative stress induces obesity and metabolic syndrome, accompanied by and likely due to exercise intolerance, vascular inflammation, and augmented adipogenesis. These data indicate that vascular ROS may play a causal role in the development of obesity and metabolic syndrome.
© 2014 by the American Diabetes Association.
Figures

Comment in
-
Oxidative stress and obesity: the chicken or the egg?Diabetes. 2014 Jul;63(7):2216-8. doi: 10.2337/db14-0424. Diabetes. 2014. PMID: 24962921 No abstract available.
Similar articles
-
The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology.Antioxid Redox Signal. 2019 Oct 1;31(10):687-709. doi: 10.1089/ars.2018.7674. Antioxid Redox Signal. 2019. PMID: 31250671 Free PMC article. Review.
-
Smooth muscle specific overexpression of p22phox potentiates carotid artery wall thickening in response to injury.Oxid Med Cell Longev. 2015;2015:305686. doi: 10.1155/2015/305686. Epub 2015 Apr 5. Oxid Med Cell Longev. 2015. PMID: 25945151 Free PMC article.
-
Oxidative stress and obesity: the chicken or the egg?Diabetes. 2014 Jul;63(7):2216-8. doi: 10.2337/db14-0424. Diabetes. 2014. PMID: 24962921 No abstract available.
-
Nox2 mediates high fat high sucrose diet-induced nitric oxide dysfunction and inflammation in aortic smooth muscle cells.J Mol Cell Cardiol. 2014 Jul;72:56-63. doi: 10.1016/j.yjmcc.2014.02.019. Epub 2014 Mar 11. J Mol Cell Cardiol. 2014. PMID: 24631774 Free PMC article.
-
Obesity and oxidative stress: potential roles of melatonin as antioxidant and metabolic regulator.Endocr Metab Immune Disord Drug Targets. 2014;14(3):159-68. doi: 10.2174/1871530314666140604151452. Endocr Metab Immune Disord Drug Targets. 2014. PMID: 24934925 Review.
Cited by
-
Nitric oxide and mitochondria in metabolic syndrome.Front Physiol. 2015 Feb 17;6:20. doi: 10.3389/fphys.2015.00020. eCollection 2015. Front Physiol. 2015. PMID: 25741283 Free PMC article. Review.
-
Vutiglabridin Alleviates Cellular Senescence with Metabolic Regulation and Circadian Clock in Human Dermal Fibroblasts.Antioxidants (Basel). 2024 Jan 16;13(1):109. doi: 10.3390/antiox13010109. Antioxidants (Basel). 2024. PMID: 38247533 Free PMC article.
-
The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology.Antioxid Redox Signal. 2019 Oct 1;31(10):687-709. doi: 10.1089/ars.2018.7674. Antioxid Redox Signal. 2019. PMID: 31250671 Free PMC article. Review.
-
Obesity and the cardiovascular health effects of fine particulate air pollution.Obesity (Silver Spring). 2014 Jul;22(7):1580-9. doi: 10.1002/oby.20748. Epub 2014 Mar 27. Obesity (Silver Spring). 2014. PMID: 24639433 Free PMC article. Review.
-
Reductive Stress Causes Pathological Cardiac Remodeling and Diastolic Dysfunction.Antioxid Redox Signal. 2020 Jun;32(18):1293-1312. doi: 10.1089/ars.2019.7808. Antioxid Redox Signal. 2020. PMID: 32064894 Free PMC article.
References
-
- Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab 1991;73:637–643 - PubMed
-
- Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab 2007;9:813–839 - PubMed
-
- Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science 2007;318:928–929 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL039006/HL/NHLBI NIH HHS/United States
- R01 HL108701/HL/NHLBI NIH HHS/United States
- T32 GM007569/GM/NIGMS NIH HHS/United States
- HL-088975/HL/NHLBI NIH HHS/United States
- HL-119968/HL/NHLBI NIH HHS/United States
- R01 HL119968/HL/NHLBI NIH HHS/United States
- HL-108701/HL/NHLBI NIH HHS/United States
- P01 GM015431/GM/NIGMS NIH HHS/United States
- HL-077440/HL/NHLBI NIH HHS/United States
- R01 HL101228/HL/NHLBI NIH HHS/United States
- R01 HL088975/HL/NHLBI NIH HHS/United States
- R01 HL077440/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

