ATP turnover by individual myosin molecules hints at two conformers of the myosin active site
- PMID: 24550279
- PMCID: PMC3932867
- DOI: 10.1073/pnas.1316390111
ATP turnover by individual myosin molecules hints at two conformers of the myosin active site
Abstract
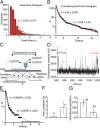
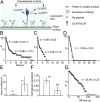
Coupling of ATP hydrolysis to structural changes in the motor domain is fundamental to the driving of motile functions by myosins. Current understanding of this chemomechanical coupling is primarily based on ensemble average measurements in solution and muscle fibers. Although important, the averaging could potentially mask essential details of the chemomechanical coupling, particularly for mixed populations of molecules. Here, we demonstrate the potential of studying individual myosin molecules, one by one, for unique insights into established systems and to dissect mixed populations of molecules where separation can be particularly challenging. We measured ATP turnover by individual myosin molecules, monitoring appearance and disappearance of fluorescent spots upon binding/dissociation of a fluorescent nucleotide to/from the active site of myosin. Surprisingly, for all myosins tested, we found two populations of fluorescence lifetimes for individual myosin molecules, suggesting that termination of fluorescence occurred by two different paths, unexpected from standard kinetic schemes of myosin ATPase. In addition, molecules of the same myosin isoform showed substantial intermolecular variability in fluorescence lifetimes. From kinetic modeling of our two fluorescence lifetime populations and earlier solution data, we propose two conformers of the active site of myosin, one that allows the complete ATPase cycle and one that dissociates ATP uncleaved. Statistical analysis and Monte Carlo simulations showed that the intermolecular variability in our studies is essentially due to the stochastic behavior of enzyme kinetics and the limited number of ATP binding events detectable from an individual myosin molecule with little room for static variation among individual molecules, previously described for other enzymes.
Keywords: ATP dwell times; TIRF microscopy; dwell time distribution; single ATP turnover assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971;10(25):4617–4624. - PubMed
-
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164(3886):1356–1365. - PubMed
-
- Johnson KA, Taylor EW. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: Reevaluation of the mechanism. Biochemistry. 1978;17(17):3432–3442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

