Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue
- PMID: 24550301
- PMCID: PMC3932915
- DOI: 10.1073/pnas.1317454111
Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue
Abstract
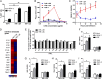
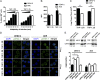
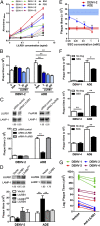
Viruses must evade the host innate defenses for replication and dengue is no exception. During secondary infection with a heterologous dengue virus (DENV) serotype, DENV is opsonized with sub- or nonneutralizing antibodies that enhance infection of monocytes, macrophages, and dendritic cells via the Fc-gamma receptor (FcγR), a process termed antibody-dependent enhancement of DENV infection. However, this enhancement of DENV infection is curious as cross-linking of activating FcγRs signals an early antiviral response by inducing the type-I IFN-stimulated genes (ISGs). Entry through activating FcγR would thus place DENV in an intracellular environment unfavorable for enhanced replication. Here we demonstrate that, to escape this antiviral response, antibody-opsonized DENV coligates leukocyte Ig-like receptor-B1 (LILRB1) to inhibit FcγR signaling for ISG expression. This immunoreceptor tyrosine-based inhibition motif-bearing receptor recruits Src homology phosphatase-1 to dephosphorylate spleen tyrosine kinase (Syk). As Syk is a key intermediate of FcγR signaling, LILRB1 coligation resulted in reduced ISG expression for enhanced DENV replication. Our findings suggest a unique mechanism for DENV to evade an early antiviral response for enhanced infection.
Keywords: early innate immune response; immune evasion; innate immune signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
LILR-B1 blocks activating FcγR signaling to allow antibody dependent enhancement of dengue virus infection.Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2404-5. doi: 10.1073/pnas.1324286111. Epub 2014 Feb 5. Proc Natl Acad Sci U S A. 2014. PMID: 24501131 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

