Therapeutic management of hypophosphatemic rickets from infancy to adulthood
- PMID: 24550322
- PMCID: PMC3959730
- DOI: 10.1530/EC-13-0103
Therapeutic management of hypophosphatemic rickets from infancy to adulthood
Abstract
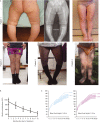
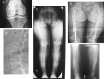
In children, hypophosphatemic rickets (HR) is revealed by delayed walking, waddling gait, leg bowing, enlarged cartilages, bone pain, craniostenosis, spontaneous dental abscesses, and growth failure. If undiagnosed during childhood, patients with hypophosphatemia present with bone and/or joint pain, fractures, mineralization defects such as osteomalacia, entesopathy, severe dental anomalies, hearing loss, and fatigue. Healing rickets is the initial endpoint of treatment in children. Therapy aims at counteracting consequences of FGF23 excess, i.e. oral phosphorus supplementation with multiple daily intakes to compensate for renal phosphate wasting and active vitamin D analogs (alfacalcidol or calcitriol) to counter the 1,25-diOH-vitamin D deficiency. Corrective surgeries for residual leg bowing at the end of growth are occasionally performed. In absence of consensus regarding indications of the treatment in adults, it is generally accepted that medical treatment should be reinitiated (or maintained) in symptomatic patients to reduce pain, which may be due to bone microfractures and/or osteomalacia. In addition to the conventional treatment, optimal care of symptomatic patients requires pharmacological and non-pharmacological management of pain and joint stiffness, through appropriated rehabilitation. Much attention should be given to the dental and periodontal manifestations of HR. Besides vitamin D analogs and phosphate supplements that improve tooth mineralization, rigorous oral hygiene, active endodontic treatment of root abscesses and preventive protection of teeth surfaces are recommended. Current outcomes of this therapy are still not optimal, and therapies targeting the pathophysiology of the disease, i.e. FGF23 excess, are desirable. In this review, medical, dental, surgical, and contributions of various expertises to the treatment of HR are described, with an effort to highlight the importance of coordinated care.
Keywords: X-linked hypophosphatemic rickets; bone; calcium; rare diseases/syndromes.
Figures



References
-
- Bacchetta J, Sea JL, Chun RF, Lisse TS, Wesseling-Perry K, Gales B, Adams JS, Salusky IB, Hewison M. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1,25-dihydroxyvitamin D in human monocytes. Journal of Bone and Mineral Research. 2013;28:46–55. doi: 10.1002/jbmr.1740. - DOI - PMC - PubMed
-
- Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. PNAS. 2011;108:E1146–E1155. doi: 10.1073/pnas.1110905108. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

