Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks
- PMID: 24550385
- PMCID: PMC3979411
- DOI: 10.1074/jbc.M113.506790
Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks
Abstract
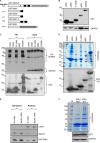
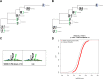
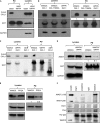
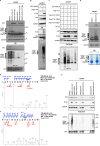
WW domains are small modules present in regulatory and signaling proteins that mediate specific protein-protein interactions. The WW domain-containing oxidoreductase (WWOX) encodes a 46-kDa tumor suppressor that contains two N-terminal WW domains and a central short-chain dehydrogenase/reductase domain. Based on its ligand recognition motifs, the WW domain family is classified into four groups. The largest one, to which WWOX belongs, recognizes ligands with a PPXY motif. To pursue the functional properties of the WW domains of WWOX, we employed mass spectrometry and phage display experiments to identify putative WWOX-interacting partners. Our analysis revealed that the first WW (WW1) domain of WWOX is the main functional interacting domain. Furthermore, our study uncovered well known and new PPXY-WW1-interacting partners and shed light on novel LPXY-WW1-interacting partners of WWOX. Many of these proteins are components of multiprotein complexes involved in molecular processes, including transcription, RNA processing, tight junction, and metabolism. By utilizing GST pull-down and immunoprecipitation assays, we validated that WWOX is a substrate of the E3 ubiquitin ligase ITCH, which contains two LPXY motifs. We found that ITCH mediates Lys-63-linked polyubiquitination of WWOX, leading to its nuclear localization and increased cell death. Our data suggest that the WW1 domain of WWOX provides a versatile platform that links WWOX with individual proteins associated with physiologically important networks.
Keywords: E3 Ubiquitin Ligase; Itch; Mass Spectrometry (MS); Protein-Protein Interactions; Tumor Suppressor Gene; Ubiquitination; WW Domain; WWOX.
Figures



References
-
- Bork P., Sudol M. (1994) The WW domain: a signalling site in dystrophin? Trends Biochem. Sci. 19, 531–533 - PubMed
-
- Sudol M., Bork P., Einbond A., Kastury K., Druck T., Negrini M., Huebner K., Lehman D. (1995) Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J. Biol. Chem. 270, 14733–14741 - PubMed
-
- Sudol M., Chen H. I., Bougeret C., Einbond A., Bork P. (1995) Characterization of a novel protein-binding module: the WW domain. FEBS Lett. 369, 67–71 - PubMed
-
- Macias M. J., Hyvönen M., Baraldi E., Schultz J., Sudol M., Saraste M., Oschkinat H. (1996) Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide. Nature 382, 646–649 - PubMed
-
- Sudol M. (1996) Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 65, 113–132 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

