Platelets support extracellular sialylation by supplying the sugar donor substrate
- PMID: 24550397
- PMCID: PMC3979374
- DOI: 10.1074/jbc.C113.546713
Platelets support extracellular sialylation by supplying the sugar donor substrate
Abstract
Sizable pools of freely circulating glycosyltransferases are in blood, but understanding their physiologic contributions has been hampered because functional sources of sugar donor substrates needed to drive extracellular glycosylation have not been identified. The blood-borne ST6Gal-1 produced and secreted by the liver is the most noted among the circulatory glycosyltransferases, and decorates marrow hematopoietic progenitor cells with α2,6-linked sialic acids and restricts blood cell production. Platelets, upon activation, secrete a plethora of bioactive molecules including pro- and anti-inflammatory mediators. Cargos of sugar donor substrates for glycosyltransferase activity have also been reported in platelets. Here, we implemented a cell-based system to interrogate platelets for their ability to deliver effectively the sugar donor substrate for extracellular ST6Gal-1 to function. We report that thrombin-activated platelets, at physiologic concentration and pH, can efficiently and effectively substitute for CMP-sialic acid in extracellular ST6Gal-1-mediated sialylation of target cell surfaces. Activated platelets can also supply the sialic acid donor to sialylate the synthetic acceptor, Gal(β1,4)GlcNAcα-o-benzyl, with the product Sia(α2,6)Gal(β1,4)GlcNAcα-o-benzyl structurally confirmed by LC/MS. Platelet-secreted donor substrate was recovered in the 100,000 × g sediment, strongly suggesting the association of this otherwise soluble substrate, putatively CMP-sialic acid, within platelet microparticles. Sequestration within microparticles may facilitate delivery of glycosylation substrate at effective dosages to sites of extracellular glycosylation while minimizing excessive dilution.
Keywords: Cell Surface; Glycosylation; Plasma; Platelets; Serum; Sialyltransferase.
Figures
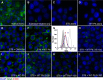
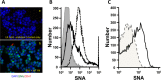

References
-
- Kaplan H. A., Woloski B. M., Hellman M., Jamieson J. C. (1983) Studies on the effect of inflammation on rat liver and serum sialyltransferase: evidence that inflammation causes release of Galβ1→4GlcNAc α2→6 sialyltransferase from liver. J. Biol. Chem. 258, 11505–11509 - PubMed
-
- Dalziel M., Lemaire S., Ewing J., Kobayashi L., Lau J. T. Y. (1999) Hepatic acute phase induction of murine β-galactoside α2,6-sialyltransferase (ST6Gal I) is IL-6 dependent and mediated by elevation of exon H-containing class of transcripts. Glycobiology 9, 1003–1008 - PubMed
-
- Jamieson J. C., Lammers G., Janzen R., Woloski B. M. (1987) The acute phase response to inflammation: the role of monokines in changes in liver glycoproteins and enzymes of glycoprotein metabolism. Comp. Biochem. Physiol. B. 87, 11–15 - PubMed
-
- Bernacki R. J., Kim U. (1977) Concomitant elevations in serum sialytransferase activity and sialic acid content in rats with metastasizing mammary tumors. Science 195, 577–580 - PubMed
-
- Nasirikenari M., Chandrasekaran E. V., Matta K. L., Segal B. H., Bogner P. N., Lugade A. A., Thanavala Y., Lee J. J., Lau J. T. (2010) Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 87, 457–466 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

