Modelling individual differences in the form of Pavlovian conditioned approach responses: a dual learning systems approach with factored representations
- PMID: 24550719
- PMCID: PMC3923662
- DOI: 10.1371/journal.pcbi.1003466
Modelling individual differences in the form of Pavlovian conditioned approach responses: a dual learning systems approach with factored representations
Abstract
Reinforcement Learning has greatly influenced models of conditioning, providing powerful explanations of acquired behaviour and underlying physiological observations. However, in recent autoshaping experiments in rats, variation in the form of Pavlovian conditioned responses (CRs) and associated dopamine activity, have questioned the classical hypothesis that phasic dopamine activity corresponds to a reward prediction error-like signal arising from a classical Model-Free system, necessary for Pavlovian conditioning. Over the course of Pavlovian conditioning using food as the unconditioned stimulus (US), some rats (sign-trackers) come to approach and engage the conditioned stimulus (CS) itself - a lever - more and more avidly, whereas other rats (goal-trackers) learn to approach the location of food delivery upon CS presentation. Importantly, although both sign-trackers and goal-trackers learn the CS-US association equally well, only in sign-trackers does phasic dopamine activity show classical reward prediction error-like bursts. Furthermore, neither the acquisition nor the expression of a goal-tracking CR is dopamine-dependent. Here we present a computational model that can account for such individual variations. We show that a combination of a Model-Based system and a revised Model-Free system can account for the development of distinct CRs in rats. Moreover, we show that revising a classical Model-Free system to individually process stimuli by using factored representations can explain why classical dopaminergic patterns may be observed for some rats and not for others depending on the CR they develop. In addition, the model can account for other behavioural and pharmacological results obtained using the same, or similar, autoshaping procedures. Finally, the model makes it possible to draw a set of experimental predictions that may be verified in a modified experimental protocol. We suggest that further investigation of factored representations in computational neuroscience studies may be useful.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

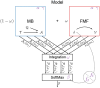
 and a value function
and a value function  values for actions
values for actions  given a state
given a state  . These values are integrated in
. These values are integrated in  , prior to be used into an action selection mechanism. The various elements may rely on parameters (in purple). The impact of flupentixol on dopamine is represented by a parameter
, prior to be used into an action selection mechanism. The various elements may rely on parameters (in purple). The impact of flupentixol on dopamine is represented by a parameter  that influences the action selection mechanism and/or any reward prediction error that might be computed in the model.
that influences the action selection mechanism and/or any reward prediction error that might be computed in the model.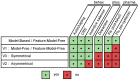

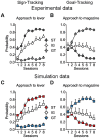
 S.E.M. and illustrated in 50-trial (2-session) blocks. (A,B) Reproduction of Flagel et al. experimental results (Figure 2 A,B). Sign-trackers (ST) made the most lever presses (black), goal-trackers (GT) made the least lever presses (white), Intermediate group (IG) is in between (grey). (C,D) Simulation of the same procedure (squares) with the model. Simulated groups of rats are defined as STs (
S.E.M. and illustrated in 50-trial (2-session) blocks. (A,B) Reproduction of Flagel et al. experimental results (Figure 2 A,B). Sign-trackers (ST) made the most lever presses (black), goal-trackers (GT) made the least lever presses (white), Intermediate group (IG) is in between (grey). (C,D) Simulation of the same procedure (squares) with the model. Simulated groups of rats are defined as STs ( ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ; n = 14) in red, GTs (
; n = 14) in red, GTs ( ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ; n = 14) in blue and IGs (
; n = 14) in blue and IGs ( ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ;
;  ; n = 14) in white. The model reproduces the same behavioural tendencies. With training, STs tend to engage more and more with the lever and less with the magazine, while GTs neglect the lever to increasingly engage with the magazine. IGs are in between.
; n = 14) in white. The model reproduces the same behavioural tendencies. With training, STs tend to engage more and more with the lever and less with the magazine, while GTs neglect the lever to increasingly engage with the magazine. IGs are in between.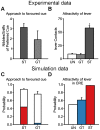
 S.E.M. Simulated groups of rats are defined as in Figure 5. (A) Number of nibbles and sniffs of preferred cue by STs and GTs as a measure for incentive salience. Data extracted from Mahler et al. from Figure 3 (bottom-left). (B) Reproduction of Robinson et al. experimental results (Figure 2 B). Lever contacts by STs and GTs during a conditioned reinforcer experiment. (C) Probability to engage with the respective favoured stimuli of STs and GTs at the end of the simulation (white, similar to the last session of Figure 5 C for STs and D for GTs) superimposed with the contribution in percentage of the values attributed by the Feature-Model-Free system in such engagement for STs (red) and GTs (blue). We hypothesize that such value is the source of incentive salience and explains why STs and GTs have a consumption-like behaviour towards their favoured stimulus. (D) Probability to engage with the lever versus exploring when presented with the lever and no magazine for STs (red), GTs (blue) and a random-policy group UN (white), simulating the unpaired group (UN) of the experimental data. Probabilities were computed by applying the softmax function after removing the values for the magazine interactions (see Methods). STs would hence actively seek to engage with the lever relatively to GTs in a Conditioned Reinforcement Effect procedure.
S.E.M. Simulated groups of rats are defined as in Figure 5. (A) Number of nibbles and sniffs of preferred cue by STs and GTs as a measure for incentive salience. Data extracted from Mahler et al. from Figure 3 (bottom-left). (B) Reproduction of Robinson et al. experimental results (Figure 2 B). Lever contacts by STs and GTs during a conditioned reinforcer experiment. (C) Probability to engage with the respective favoured stimuli of STs and GTs at the end of the simulation (white, similar to the last session of Figure 5 C for STs and D for GTs) superimposed with the contribution in percentage of the values attributed by the Feature-Model-Free system in such engagement for STs (red) and GTs (blue). We hypothesize that such value is the source of incentive salience and explains why STs and GTs have a consumption-like behaviour towards their favoured stimulus. (D) Probability to engage with the lever versus exploring when presented with the lever and no magazine for STs (red), GTs (blue) and a random-policy group UN (white), simulating the unpaired group (UN) of the experimental data. Probabilities were computed by applying the softmax function after removing the values for the magazine interactions (see Methods). STs would hence actively seek to engage with the lever relatively to GTs in a Conditioned Reinforcement Effect procedure.
 S.E.M. (A,B) Reproduction of Flagel et al. experimental results (Figure 3 d,f). Phasic dopamine release recorded in the core of the nucleus accumbens in STs (light grey) and GTs (grey) using Fast Scan Cyclic Voltammetry. Change in peak amplitude of the dopamine signal observed in response to CS and US presentation for each session of conditioning (C,D) Average RPE computed by the Feature-Model-Free system in response to CS and US presentation for each session of conditioning. Simulated groups of rats are defined as in Figure 5. The model is able to qualitatively reproduce the physiological data. STs (blue) show a shift of activity from US to CS time over training, while GTs develop a second activity at CS time while maintaining the initial activity at US time.
S.E.M. (A,B) Reproduction of Flagel et al. experimental results (Figure 3 d,f). Phasic dopamine release recorded in the core of the nucleus accumbens in STs (light grey) and GTs (grey) using Fast Scan Cyclic Voltammetry. Change in peak amplitude of the dopamine signal observed in response to CS and US presentation for each session of conditioning (C,D) Average RPE computed by the Feature-Model-Free system in response to CS and US presentation for each session of conditioning. Simulated groups of rats are defined as in Figure 5. The model is able to qualitatively reproduce the physiological data. STs (blue) show a shift of activity from US to CS time over training, while GTs develop a second activity at CS time while maintaining the initial activity at US time.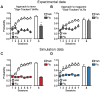
 S.E.M. (A,B) Reproduction of Flagel et al. experimental results (Figure 4 a,d). Effects of flupentixol on the probability to approach the lever for STs (A) and the magazine for GTs (B) during lever presentation. (C,D) Simulation of the same procedure (squares) with the model. Simulated groups of rats are defined as in Figure 5. (C) By flattening the softmax temperature and reducing the RPEs of the Feature-Model-Free system, to mimic the possible effect of flupentixol, the model can reproduce the blocked acquisition of sign-tracking in STs (red), engaging less the lever relatively to a saline-injected control group (white). (D) Similarly, the model reproduces that goal-tracking was learned but its expression was blocked. Under flupentixol (first 7 sessions), GTs (blue) did not express goal-tracking, but on a flupentixol-free control test (
S.E.M. (A,B) Reproduction of Flagel et al. experimental results (Figure 4 a,d). Effects of flupentixol on the probability to approach the lever for STs (A) and the magazine for GTs (B) during lever presentation. (C,D) Simulation of the same procedure (squares) with the model. Simulated groups of rats are defined as in Figure 5. (C) By flattening the softmax temperature and reducing the RPEs of the Feature-Model-Free system, to mimic the possible effect of flupentixol, the model can reproduce the blocked acquisition of sign-tracking in STs (red), engaging less the lever relatively to a saline-injected control group (white). (D) Similarly, the model reproduces that goal-tracking was learned but its expression was blocked. Under flupentixol (first 7 sessions), GTs (blue) did not express goal-tracking, but on a flupentixol-free control test ( session) their engagement with the magazine was almost identical to the engagement of a saline-injected control group (white).
session) their engagement with the magazine was almost identical to the engagement of a saline-injected control group (white).
 S.E.M. (A,B) Reproduction of Saunders et al. experimental results (Figure 2 A,D). Effects of different doses of flupentixol on the general tendency to sign-track (A) and goal-track (B) in a population of rats, without discriminating between sign- and goal-trackers. (C,D) Simulation of the same procedure with the model. The simulated population is composed of groups of rats defined as in Figure 5. By simulating the effect of flupentixol as in Figure 8, the model is able to reproduce the decreasing tendency to sign-track in the overall population by increasing the dose of flupentixol.
S.E.M. (A,B) Reproduction of Saunders et al. experimental results (Figure 2 A,D). Effects of different doses of flupentixol on the general tendency to sign-track (A) and goal-track (B) in a population of rats, without discriminating between sign- and goal-trackers. (C,D) Simulation of the same procedure with the model. The simulated population is composed of groups of rats defined as in Figure 5. By simulating the effect of flupentixol as in Figure 8, the model is able to reproduce the decreasing tendency to sign-track in the overall population by increasing the dose of flupentixol.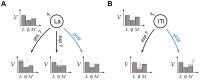
 based on features. Choosing an action (e.g. goL, goM or exp), defines the feature it is focusing on (e.g. Lever, Magazine or nothing
based on features. Choosing an action (e.g. goL, goM or exp), defines the feature it is focusing on (e.g. Lever, Magazine or nothing  ). Once the action is chosen (e.g. goM in blue), only the value of the focused feature (e.g.
). Once the action is chosen (e.g. goM in blue), only the value of the focused feature (e.g.  ) is updated by a standard reward prediction error, while leaving the values of the other features unchanged. (B) Feature-values permit generalization. At a different place and time in the episode, the agent can choose an action (e.g. goM in blue) focusing on a feature (e.g. M) that might have already been focused on. This leads to the revision of the same value (e.g.
) is updated by a standard reward prediction error, while leaving the values of the other features unchanged. (B) Feature-values permit generalization. At a different place and time in the episode, the agent can choose an action (e.g. goM in blue) focusing on a feature (e.g. M) that might have already been focused on. This leads to the revision of the same value (e.g.  ) for two different states (e.g.
) for two different states (e.g.  and
and  ). Values of features are shared amongst multiple states.
). Values of features are shared amongst multiple states.
References
-
- Sutton RS, Barto AG (1998) Reinforcement learning: An introduction. The MIT Press.
-
- Sutton RS, Barto AG (1987) A temporal-difference model of classical conditioning. In: Proceedings of the ninth annual conference of the cognitive science society. Seattle, WA, pp. 355–378.
-
- Barto AG (1995) Adaptive critics and the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors, Models of information processing in the basal ganglia, The MIT Press. pp. 215–232.
-
- Simon DA, Daw ND (2012) Dual-system learning models and drugs of abuse. In: Computational Neuroscience of Drug Addiction, Springer. pp. 145–161.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

