CIB1 synergizes with EphrinA2 to regulate Kaposi's sarcoma-associated herpesvirus macropinocytic entry in human microvascular dermal endothelial cells
- PMID: 24550731
- PMCID: PMC3923796
- DOI: 10.1371/journal.ppat.1003941
CIB1 synergizes with EphrinA2 to regulate Kaposi's sarcoma-associated herpesvirus macropinocytic entry in human microvascular dermal endothelial cells
Abstract
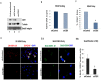
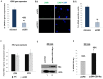
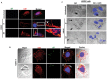
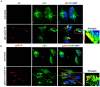
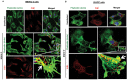
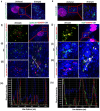
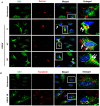
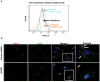
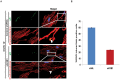
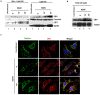
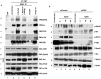
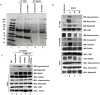
KSHV envelope glycoproteins interact with cell surface heparan sulfate and integrins, and activate FAK, Src, PI3-K, c-Cbl, and Rho-GTPase signal molecules in human microvascular dermal endothelial (HMVEC-d) cells. c-Cbl mediates the translocation of virus bound α3β1 and αVβ3 integrins into lipid rafts (LRs), where KSHV interacts and activates EphrinA2 (EphA2). EphA2 associates with c-Cbl-myosin IIA and augmented KSHV-induced Src and PI3-K signals in LRs, leading to bleb formation and macropinocytosis of KSHV. To identify the factor(s) coordinating the EphA2-signal complex, the role of CIB1 (calcium and integrin binding protein-1) associated with integrin signaling was analyzed. CIB1 knockdown did not affect KSHV binding to HMVEC-d cells but significantly reduced its entry and gene expression. In contrast, CIB1 overexpression increased KSHV entry in 293 cells. Single virus particle infection and trafficking during HMVEC-d cell entry was examined by utilizing DiI (envelope) and BrdU (viral DNA) labeled virus. CIB1 was associated with KSHV in membrane blebs and in Rab5 positive macropinocytic vesicles. CIB1 knockdown abrogated virus induced blebs, macropinocytosis and virus association with the Rab5 macropinosome. Infection increased the association of CIB1 with LRs, and CIB1 was associated with EphA2 and KSHV entry associated signal molecules such as Src, PI3-K, and c-Cbl. CIB1 knockdown significantly reduced the infection induced EphA2, Src and Erk1/2 activation. Mass spectrometry revealed the simultaneous association of CIB1 and EphA2 with the actin cytoskeleton modulating myosin IIA and alpha-actinin 4 molecules, and CIB1 knockdown reduced EphA2's association with myosin IIA and alpha-actinin 4. Collectively, these studies revealed for the first time that CIB1 plays a role in virus entry and macropinocytosis, and suggested that KSHV utilizes CIB1 as one of the key molecule(s) to coordinate and sustain the EphA2 mediated signaling involved in its entry, and CIB1 is an attractive therapeutic target to block KSHV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266: 1865–1869. - PubMed
-
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM (1995) Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332: 1186–1191. - PubMed
-
- Ganem D (2007) Kaposi's sarcoma-associated herpesvirus. Fields virology 5th ed., vol. 2: p. 2875–2888.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

