Male-killing Spiroplasma induces sex-specific cell death via host apoptotic pathway
- PMID: 24550732
- PMCID: PMC3923752
- DOI: 10.1371/journal.ppat.1003956
Male-killing Spiroplasma induces sex-specific cell death via host apoptotic pathway
Abstract
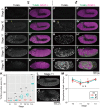
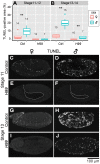
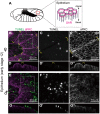
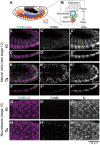
Some symbiotic bacteria cause remarkable reproductive phenotypes like cytoplasmic incompatibility and male-killing in their host insects. Molecular and cellular mechanisms underlying these symbiont-induced reproductive pathologies are of great interest but poorly understood. In this study, Drosophila melanogaster and its native Spiroplasma symbiont strain MSRO were investigated as to how the host's molecular, cellular and morphogenetic pathways are involved in the symbiont-induced male-killing during embryogenesis. TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining, anti-cleaved-Caspase-3 antibody staining, and apoptosis-deficient mutant analysis unequivocally demonstrated that the host's apoptotic pathway is involved in Spiroplasma-induced male-specific embryonic cell death. Double-staining with TUNEL and an antibody recognizing epidermal marker showed that embryonic epithelium is the main target of Spiroplasma-induced male-specific apoptosis. Immunostaining with antibodies against markers of differentiated and precursor neural cells visualized severe neural defects specifically in Spiroplasma-infected male embryos as reported in previous studies. However, few TUNEL signals were detected in the degenerate nervous tissues of male embryos, and the Spiroplasma-induced neural defects in male embryos were not suppressed in an apoptosis-deficient host mutant. These results suggest the possibility that the apoptosis-dependent epidermal cell death and the apoptosis-independent neural malformation may represent different mechanisms underlying the Spiroplasma-induced male-killing. Despite the male-specific progressive embryonic abnormality, Spiroplasma titers remained almost constant throughout the observed stages of embryonic development and across male and female embryos. Strikingly, a few Spiroplasma-infected embryos exhibited gynandromorphism, wherein apoptotic cell death was restricted to male cells. These observations suggest that neither quantity nor proliferation of Spiroplasma cells but some Spiroplasma-derived factor(s) may be responsible for the expression of the male-killing phenotype.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Buchner P (1965) Endosymbiosis of Animals with Plant Microorganisms. New York, NY, USA: Interscience Publishers. 909 p.
-
- Bourtzis K, Miller TA (2003) Insect Symbiosis. Boca Raton, FL: CRC Press. 156 p.
-
- Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190 doi:10.1146/annurev.genet.41.110306.130119 - DOI - PubMed
-
- Ohkuma M (2003) Termite symbiotic systems: efficient bio-recycling of lignocellulose. Appl Microbiol Biotechnol 61: 1–9 doi:10.1007/s00253-002-1189-z - DOI - PubMed
-
- Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55: 247–266 doi:10.1146/annurev-ento-112408-085305 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

