Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma
- PMID: 24550739
- PMCID: PMC3923676
- DOI: 10.1371/journal.pgen.1004135
Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma
Abstract
Advanced cholangiocarcinoma continues to harbor a difficult prognosis and therapeutic options have been limited. During the course of a clinical trial of whole genomic sequencing seeking druggable targets, we examined six patients with advanced cholangiocarcinoma. Integrated genome-wide and whole transcriptome sequence analyses were performed on tumors from six patients with advanced, sporadic intrahepatic cholangiocarcinoma (SIC) to identify potential therapeutically actionable events. Among the somatic events captured in our analysis, we uncovered two novel therapeutically relevant genomic contexts that when acted upon, resulted in preliminary evidence of anti-tumor activity. Genome-wide structural analysis of sequence data revealed recurrent translocation events involving the FGFR2 locus in three of six assessed patients. These observations and supporting evidence triggered the use of FGFR inhibitors in these patients. In one example, preliminary anti-tumor activity of pazopanib (in vitro FGFR2 IC50≈350 nM) was noted in a patient with an FGFR2-TACC3 fusion. After progression on pazopanib, the same patient also had stable disease on ponatinib, a pan-FGFR inhibitor (in vitro, FGFR2 IC50≈8 nM). In an independent non-FGFR2 translocation patient, exome and transcriptome analysis revealed an allele specific somatic nonsense mutation (E384X) in ERRFI1, a direct negative regulator of EGFR activation. Rapid and robust disease regression was noted in this ERRFI1 inactivated tumor when treated with erlotinib, an EGFR kinase inhibitor. FGFR2 fusions and ERRFI mutations may represent novel targets in sporadic intrahepatic cholangiocarcinoma and trials should be characterized in larger cohorts of patients with these aberrations.
Conflict of interest statement
Dr. Kipp received grant funding from Abbott Molecular Inc, the suppliers of the FGFR2 FISH probe used in this study. The other authors have no conflicts to declare.
Figures

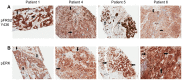
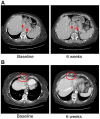
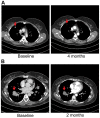
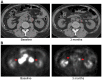

References
-
- Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, et al. (1996) Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. International journal of epidemiology 25: 933–940. - PubMed
-
- Watanapa P (1996) Cholangiocarcinoma in patients with opisthorchiasis. The British journal of surgery 83: 1062–1064. - PubMed
-
- Watanapa P, Watanapa WB (2002) Liver fluke-associated cholangiocarcinoma. The British journal of surgery 89: 962–970. - PubMed
-
- Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, et al. (2002) Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. Journal of hepatology 36: 321–327. - PubMed
-
- Bergquist A, Glaumann H, Persson B, Broome U (1998) Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology 27: 311–316. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

