Accurate and robust genomic prediction of celiac disease using statistical learning
- PMID: 24550740
- PMCID: PMC3923679
- DOI: 10.1371/journal.pgen.1004137
Accurate and robust genomic prediction of celiac disease using statistical learning
Erratum in
- PLoS Genet. 2014 Apr;10(4):e1004374
Abstract
Practical application of genomic-based risk stratification to clinical diagnosis is appealing yet performance varies widely depending on the disease and genomic risk score (GRS) method. Celiac disease (CD), a common immune-mediated illness, is strongly genetically determined and requires specific HLA haplotypes. HLA testing can exclude diagnosis but has low specificity, providing little information suitable for clinical risk stratification. Using six European cohorts, we provide a proof-of-concept that statistical learning approaches which simultaneously model all SNPs can generate robust and highly accurate predictive models of CD based on genome-wide SNP profiles. The high predictive capacity replicated both in cross-validation within each cohort (AUC of 0.87-0.89) and in independent replication across cohorts (AUC of 0.86-0.9), despite differences in ethnicity. The models explained 30-35% of disease variance and up to ∼43% of heritability. The GRS's utility was assessed in different clinically relevant settings. Comparable to HLA typing, the GRS can be used to identify individuals without CD with ≥99.6% negative predictive value however, unlike HLA typing, fine-scale stratification of individuals into categories of higher-risk for CD can identify those that would benefit from more invasive and costly definitive testing. The GRS is flexible and its performance can be adapted to the clinical situation by adjusting the threshold cut-off. Despite explaining a minority of disease heritability, our findings indicate a genomic risk score provides clinically relevant information to improve upon current diagnostic pathways for CD and support further studies evaluating the clinical utility of this approach in CD and other complex diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
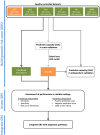
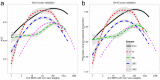



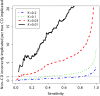
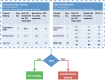
References
-
- Anderson RP (2011) Coeliac disease is on the rise. Med J Aust 194: 278–279. - PubMed
-
- Green PH, Cellier C (2007) Celiac disease. N Engl J Med 357: 1731–1743. - PubMed
-
- Catassi C, Kryszak D, Louis-Jacques O, Duerksen DR, Hill I, et al. (2007) Detection of Celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol 102: 1454–1460. - PubMed
-
- Dube C, Rostom A, Sy R, Cranney A, Saloojee N, et al. (2005) The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology 128: S57–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

