Therapeutic drug monitoring and pharmacogenetic study of HIV-infected ethnic Chinese receiving efavirenz-containing antiretroviral therapy with or without rifampicin-based anti-tuberculous therapy
- PMID: 24551111
- PMCID: PMC3925114
- DOI: 10.1371/journal.pone.0088497
Therapeutic drug monitoring and pharmacogenetic study of HIV-infected ethnic Chinese receiving efavirenz-containing antiretroviral therapy with or without rifampicin-based anti-tuberculous therapy
Abstract
Objectives: Plasma efavirenz concentrations in HIV-infected patients with tuberculosis (TB) may be affected by cytochrome P450 (CYP) 2B6 single-nucleotide polymorphisms and concurrent rifampicin use. We aimed to investigate the effects of CYP2B6 G516T polymorphisms and concomitant rifampicin use on the plasma efavirenz concentrations in HIV-infected Taiwanese.
Methods: HIV-infected patients with or without TB who had received combination antiretroviral therapy containing efavirenz (600 mg daily) for two weeks or greater were enrolled for determinations of CYP2B6 G516T polymorphism and plasma efavirenz concentrations with the use of polymerase-chain-reaction restriction fragment-length polymorphism and high-performance liquid chromatography, respectively.
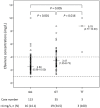
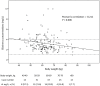
Results: From October 2009 to August 2012, 171 HIV-infected patients, including 18 with TB, were enrolled 113 (66.1%) with CYP2B6 G516G, 55 (32.2%) GT, and 3 (1.8%) TT genotype. Patients receiving rifampicin had a significantly lower median plasma efavirenz concentration than the control group (2.16 vs 2.92 mg/L, P = 0.003); however, all patients achieved target plasma concentration (>1 mg/L). Patients with GT or TT genotype had a significantly higher plasma concentration than those with GG genotype (2.50 vs 3.47 mg/L for GT genotype and 8.78 mg/L for TT genotype, P<0.001). Plasma efavirenz concentration >4 mg/L was noted in 38 (22.2%) patients, which was associated with a lower weight (per 10-kg increase, odds ratio, 0.52; 95% confidence interval, 0.33-0.83) and GT or TT genotype (odds ratio, 4.35; 95% confidence interval, 1.97-9.59) in multivariate analysis.
Conclusions: Despite combination with rifampicin, sufficient plasma efavirenz concentrations can be achieved in HIV-infected Taiwanese with TB who receive efavirenz 600 mg daily. Carriage of CYP2B6 516 GT and TT genotypes and a lower weight are associated with higher plasma efavirenz concentrations.
Conflict of interest statement
Figures

References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents (2013) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed: 16 June 2013.
-
- Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, et al. (2001) Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15: 71–75. - PubMed
-
- Burger D, van der Heiden I, la Porte C, van der Ende M, Groeneveld P, et al. (2006) Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol 61: 148–154. - PMC - PubMed
-
- Stohr W, Back D, Dunn D, Sabin C, Winston A, et al. (2008) Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther 13: 675–685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

