Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death
- PMID: 24551114
- PMCID: PMC3925142
- DOI: 10.1371/journal.pone.0088521
Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death
Abstract
Background: Livers derived from donation after circulatory death (DCD) are increasingly accepted for transplantation. However, DCD livers suffer additional donor warm ischemia, leading to biliary injury and more biliary complications after transplantation. It is unknown whether oxygenated machine perfusion results in better preservation of biliary epithelium and the peribiliary vasculature. We compared oxygenated hypothermic machine perfusion (HMP) with static cold storage (SCS) in a porcine DCD model.
Methods: After 30 min of cardiac arrest, livers were perfused in situ with HTK solution (4°C) and preserved for 4 h by either SCS (n = 9) or oxygenated HMP (10°C; n = 9), using pressure-controlled arterial and portal venous perfusion. To simulate transplantation, livers were reperfused ex vivo at 37°C with oxygenated autologous blood. Bile duct injury and function were determined by biochemical and molecular markers, and a systematic histological scoring system.
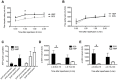
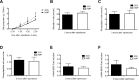
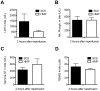
Results: After reperfusion, arterial flow was higher in the HMP group, compared to SCS (251±28 vs 166±28 mL/min, respectively, after 1 hour of reperfusion; p = 0.003). Release of hepatocellular enzymes was significantly higher in the SCS group. Markers of biliary epithelial injury (biliary LDH, gamma-GT) and function (biliary pH and bicarbonate, and biliary transporter expression) were similar in the two groups. However, histology of bile ducts revealed significantly less arteriolonecrosis of the peribiliary vascular plexus in HMP preserved livers (>50% arteriolonecrosis was observed in 7 bile ducts of the SCS preserved livers versus only 1 bile duct of the HMP preserved livers; p = 0.024).
Conclusions: Oxygenated HMP prevents arteriolonecrosis of the peribiliary vascular plexus of the bile ducts of DCD pig livers and results in higher arterial flow after reperfusion. Together this may contribute to better perfusion of the bile ducts, providing a potential advantage in the post-ischemic recovery of bile ducts.
Conflict of interest statement
Figures



References
-
- Pine JK, Aldouri A, Young AL, Davies MH, Attia M, et al. (2009) Liver transplantation following donation after cardiac death: An analysis using matched pairs. Liver Transpl 15: 1072–1082. - PubMed
-
- Meurisse N, Vanden Bussche S, Jochmans I, Francois J, Desschans B, et al. (2012) Outcomes of liver transplantations using donations after circulatory death: A single-center experience. Transplant Proc 44: 2868–2873. - PubMed
-
- Suarez F, Otero A, Solla M, Arnal F, Lorenzo MJ, et al. (2008) Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation 85: 9–14. - PubMed
-
- Dubbeld J, Hoekstra H, Farid W, Ringers J, Porte RJ, et al. (2010) Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br J Surg 97: 744–753. - PubMed
-
- Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, et al. (2007) Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl 13: 725–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

