Complement-mediated virus infectivity neutralisation by HLA antibodies is associated with sterilising immunity to SIV challenge in the macaque model for HIV/AIDS
- PMID: 24551145
- PMCID: PMC3925162
- DOI: 10.1371/journal.pone.0088735
Complement-mediated virus infectivity neutralisation by HLA antibodies is associated with sterilising immunity to SIV challenge in the macaque model for HIV/AIDS
Abstract
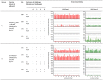
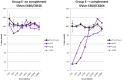
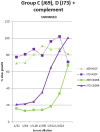
Sterilising immunity is a desired outcome for vaccination against human immunodeficiency virus (HIV) and has been observed in the macaque model using inactivated simian immunodeficiency virus (SIV). This protection was attributed to antibodies specific for cell proteins including human leucocyte antigens (HLA) class I and II incorporated into virions during vaccine and challenge virus preparation. We show here, using HLA bead arrays, that vaccinated macaques protected from virus challenge had higher serum antibody reactivity compared with non-protected animals. Moreover, reactivity was shown to be directed against HLA framework determinants. Previous studies failed to correlate serum antibody mediated virus neutralisation with protection and were confounded by cytotoxic effects. Using a virus entry assay based on TZM-bl cells we now report that, in the presence of complement, serum antibody titres that neutralise virus infectivity were higher in protected animals. We propose that complement-augmented virus neutralisation is a key factor in inducing sterilising immunity and may be difficult to achieve with HIV/SIV Env-based vaccines. Understanding how to overcome the apparent block of inactivated SIV vaccines to elicit anti-envelope protein antibodies that effectively engage the complement system could enable novel anti-HIV antibody vaccines that induce potent, virolytic serological response to be developed.
Conflict of interest statement
Figures




Similar articles
-
Analysis of Complement-Mediated Lysis of Simian Immunodeficiency Virus (SIV) and SIV-Infected Cells Reveals Sex Differences in Vaccine-Induced Immune Responses in Rhesus Macaques.J Virol. 2018 Sep 12;92(19):e00721-18. doi: 10.1128/JVI.00721-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021899 Free PMC article.
-
Immunization with recombinant HLA classes I and II, HIV-1 gp140, and SIV p27 elicits protection against heterologous SHIV infection in rhesus macaques.J Virol. 2011 Jul;85(13):6442-52. doi: 10.1128/JVI.00129-11. Epub 2011 Apr 13. J Virol. 2011. PMID: 21490092 Free PMC article.
-
Early potent protection against heterologous SIVsmE660 challenge following live attenuated SIV vaccination in Mauritian cynomolgus macaques.PLoS One. 2011;6(8):e23092. doi: 10.1371/journal.pone.0023092. Epub 2011 Aug 10. PLoS One. 2011. PMID: 21853072 Free PMC article.
-
Simian and feline immunodeficiency viruses: animal lentivirus models for evaluation of AIDS vaccines and antiviral agents.Antiviral Res. 1991 May;15(4):267-86. doi: 10.1016/0166-3542(91)90009-g. Antiviral Res. 1991. PMID: 1659310 Review.
-
SIV vaccines: current status. The role of the SIV-macaque model in AIDS research.Vaccine. 1991 Nov;9(11):787-91. doi: 10.1016/0264-410x(91)90213-p. Vaccine. 1991. PMID: 1759500 Review.
Cited by
-
Analysis of Complement-Mediated Lysis of Simian Immunodeficiency Virus (SIV) and SIV-Infected Cells Reveals Sex Differences in Vaccine-Induced Immune Responses in Rhesus Macaques.J Virol. 2018 Sep 12;92(19):e00721-18. doi: 10.1128/JVI.00721-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021899 Free PMC article.
-
Antiviral neutralizing antibodies: from in vitro to in vivo activity.Nat Rev Immunol. 2023 Nov;23(11):720-734. doi: 10.1038/s41577-023-00858-w. Epub 2023 Apr 17. Nat Rev Immunol. 2023. PMID: 37069260 Free PMC article. Review.
-
HLA antibody repertoire in infants suggests selectivity in transplacental crossing.Am J Reprod Immunol. 2020 Aug;84(2):e13264. doi: 10.1111/aji.13264. Epub 2020 Jun 16. Am J Reprod Immunol. 2020. PMID: 32395838 Free PMC article.
-
Viral Evasion of the Complement System and Its Importance for Vaccines and Therapeutics.Front Immunol. 2020 Jul 9;11:1450. doi: 10.3389/fimmu.2020.01450. eCollection 2020. Front Immunol. 2020. PMID: 32733480 Free PMC article. Review.
-
Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives.Adv Biol. 2014;2014:157895. doi: 10.1155/2014/157895. Epub 2014 Sep 9. Adv Biol. 2014. PMID: 27099867 Free PMC article.
References
-
- Karlsson Hedestam GB, Fouchier RAM, Phogat S, Burton DR, Sodroski J, et al. (2008) The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Micro 6: 143–155. - PubMed
-
- Sattentau QJ, Moulard M, Brivet B, Botto F, Guillemot JC, et al. (1999) Antibody neutralization of HIV-1 and the potential for vaccine design. Immunology Letters 66: 143–149. - PubMed
-
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, et al. (2000) Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature Medicine 6: 200–206. - PubMed
-
- Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, et al. (2008) Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22: 339–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

