Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation
- PMID: 24551161
- PMCID: PMC3923824
- DOI: 10.1371/journal.pone.0088772
Fibroblast activation protein (FAP) is essential for the migration of bone marrow mesenchymal stem cells through RhoA activation
Abstract
Background: The ability of human bone marrow mesenchymal stem cells (BM-MSCs) to migrate and localize specifically to injured tissues is central in developing therapeutic strategies for tissue repair and regeneration. Fibroblast activation protein (FAP) is a cell surface serine protease expressed at sites of tissue remodeling during embryonic development. It is also expressed in BM-MSCs, but not in normal tissues or cells. The function of FAP in BM-MSCs is not known.
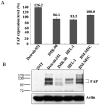
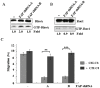
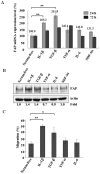
Principal findings: We found that depletion of FAP proteins significantly inhibited the migration of BM-MSCs in a transwell chemotaxis assay. Such impaired migration ability of BM-MSCs could be rescued by re-expressing FAP in these cells. We then demonstrated that depletion of FAP activated intracellular RhoA GTPase. Consistently, inhibition of RhoA activity using a RhoA inhibitor rescued its migration ability. Inhibition of FAP activity with an FAP-specific inhibitor did not affect the activation of RhoA or the migration of BM-MSCs. Furthermore, the inflammatory cytokines interleukin-1beta (IL-1β) and transforming growth factor-beta (TGF-β) upregulated FAP expression, which coincided with better BM-MSC migration.
Conclusions: Our results indicate FAP plays an important role in the migration of BM-MSCs through modulation of RhoA GTPase activity. The peptidase activity of FAP is not essential for such migration. Cytokines IL-1β and TGF-β upregulate the expression level of FAP and thus enhance BM-MSC migration.
Conflict of interest statement
Figures




Similar articles
-
Coordinated Regulation of Mesenchymal Stem Cell Migration by Various Chemotactic Stimuli.Int J Mol Sci. 2020 Nov 13;21(22):8561. doi: 10.3390/ijms21228561. Int J Mol Sci. 2020. PMID: 33202862 Free PMC article.
-
N-cadherin mediates the migration of bone marrow-derived mesenchymal stem cells toward breast tumor cells.Theranostics. 2021 May 3;11(14):6786-6799. doi: 10.7150/thno.59703. eCollection 2021. Theranostics. 2021. PMID: 34093853 Free PMC article.
-
Bone marrow-derived mesenchymal stem cells promote invasiveness and transendothelial migration of osteosarcoma cells via a mesenchymal to amoeboid transition.Mol Oncol. 2018 May;12(5):659-676. doi: 10.1002/1878-0261.12189. Epub 2018 Mar 31. Mol Oncol. 2018. PMID: 29517849 Free PMC article.
-
Targeting fibroblast activation protein in cancer - Prospects and caveats.Front Biosci (Landmark Ed). 2018 Jun 1;23(10):1933-1968. doi: 10.2741/4682. Front Biosci (Landmark Ed). 2018. PMID: 29772538 Review.
-
Fibroblast activation protein-α in fibrogenic disorders and cancer: more than a prolyl-specific peptidase?Expert Opin Ther Targets. 2017 Oct;21(10):977-991. doi: 10.1080/14728222.2017.1370455. Epub 2017 Aug 30. Expert Opin Ther Targets. 2017. PMID: 28829211 Review.
Cited by
-
The role of fibroblast activation protein in health and malignancy.Cancer Metastasis Rev. 2020 Sep;39(3):783-803. doi: 10.1007/s10555-020-09909-3. Cancer Metastasis Rev. 2020. PMID: 32601975 Free PMC article. Review.
-
Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review).Mol Med Rep. 2022 May;25(5):171. doi: 10.3892/mmr.2022.12687. Epub 2022 Mar 18. Mol Med Rep. 2022. PMID: 35302174 Free PMC article. Review.
-
Highlights of Strategies Targeting Fibroblasts for Novel Therapies for Rheumatoid Arthritis.Front Med (Lausanne). 2022 Feb 17;9:846300. doi: 10.3389/fmed.2022.846300. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35252279 Free PMC article. Review.
-
Identification of eight-protein biosignature for diagnosis of tuberculosis.Thorax. 2020 Jul;75(7):576-583. doi: 10.1136/thoraxjnl-2018-213021. Epub 2020 Mar 22. Thorax. 2020. PMID: 32201389 Free PMC article.
-
Transplantation of human matrix metalloproteinase-1 gene-modified bone marrow-derived mesenchymal stem cell attenuates CCL4-induced liver fibrosis in rats.Int J Mol Med. 2018 Jun;41(6):3175-3184. doi: 10.3892/ijmm.2018.3516. Epub 2018 Feb 27. Int J Mol Med. 2018. PMID: 29512750 Free PMC article.
References
-
- Rosenblum JS, Kozarich JW (2003) Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol 7: 496–504. - PubMed
-
- Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, et al. (2003) Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol 120: 182–188. - PubMed
-
- Chen WT, Kelly T (2003) Seprase complexes in cellular invasiveness. Cancer Metastasis Rev 22: 259–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

