Systemic delivery of siRNA down regulates brain prion protein and ameliorates neuropathology in prion disorder
- PMID: 24551164
- PMCID: PMC3925167
- DOI: 10.1371/journal.pone.0088797
Systemic delivery of siRNA down regulates brain prion protein and ameliorates neuropathology in prion disorder
Abstract
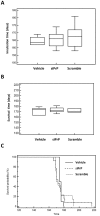
One of the main challenges for neurodegenerative disorders that are principally incurable is the development of new therapeutic strategies, which raises important medical, scientific and societal issues. Creutzfeldt-Jakob diseases are rare neurodegenerative fatal disorders which today remain incurable. The objective of this study was to evaluate the efficacy of the down-regulation of the prion protein (PrP) expression using siRNA delivered by, a water-in-oil microemulsion, as a therapeutic candidate in a preclinical study. After 12 days rectal mucosa administration of Aonys/PrP-siRNA in mice, we observed a decrease of about 28% of the brain PrP(C) level. The effect of Aonys/PrP-siRNA was then evaluated on prion infected mice. Several mice presented a delay in the incubation and survival time compared to the control groups and a significant impact was observed on astrocyte reaction and neuronal survival in the PrP-siRNA treated groups. These results suggest that a new therapeutic scheme based an innovative delivery system of PrP-siRNA can be envisioned in prion disorders.
Conflict of interest statement
Figures





References
-
- Sikorska B, Liberski PP (2012) Human prion diseases: from kuru to variant creutzfeldt-jakob disease. Subcell Biochem 65: 457–496. - PubMed
-
- Parchi P, Saverioni D (2012) Molecular pathology, classification, and diagnosis of sporadic human prion disease variants. Folia Neuropathol 50: 20–45. - PubMed
-
- Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144. - PubMed
-
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. - PubMed
-
- Sailer A, Bueler H, Fischer M, Aguzzi A, Weissmann C (1994) No propagation of prions in mice devoid of PrP. Cell 77: 967–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

