TrkB agonist antibody pretreatment enhances neuronal survival and long-term sensory motor function following hypoxic ischemic injury in neonatal rats
- PMID: 24551199
- PMCID: PMC3925177
- DOI: 10.1371/journal.pone.0088962
TrkB agonist antibody pretreatment enhances neuronal survival and long-term sensory motor function following hypoxic ischemic injury in neonatal rats
Abstract
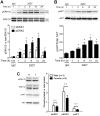
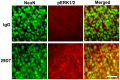
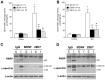
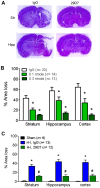
Perinatal hypoxic ischemia (H-I) causes brain damage and long-term neurological impairments, leading to motor dysfunctions and cerebral palsy. Many studies have demonstrated that the TrkB-ERK1/2 signaling pathway plays a key role in mediating the protective effect of brain-derived neurotrophic factor (BDNF) following perinatal H-I brain injury in experimental animals. In the present study, we explored the neuroprotective effects of the TrkB-specific agonist monoclonal antibody 29D7 on H-I brain injury in neonatal rats. First, we found that intracerebroventricular (icv) administration of 29D7 in normal P7 rats markedly increased the levels of phosphorylated ERK1/2 and phosphorylated AKT in neurons up to 24 h. Second, P7 rats received icv administration of 29D7 and subjected to H-I injury induced by unilateral carotid artery ligation and exposure to hypoxia (8% oxygen). We found that 29D7, to a similar extent to BDNF, significantly inhibited activation of caspase-3, a biochemical hallmark of apoptosis, following H-I injury. Third, we found that this 29D7-mediated neuroprotective action persisted at least up to 5 weeks post-H-I injury as assessed by brain tissue loss, implicating long-term neurotrophic effects rather than an acute delay of cell death. Moreover, the long-term neuroprotective effect of 29D7 was tightly correlated with sensorimotor functional recovery as assessed by a tape-removal test, while 29D7 did not significantly improve rotarod performance. Taken together, these findings demonstrate that pretreatment with the TrkB-selective agonist 29D7 significantly increases neuronal survival and behavioral recovery following neonatal hypoxic-ischemic brain injury.
Conflict of interest statement
Figures

Similar articles
-
Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells.Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1747-54. doi: 10.1167/iovs.09-4450. Epub 2009 Oct 29. Invest Ophthalmol Vis Sci. 2010. PMID: 19875669 Free PMC article.
-
BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway.J Neurosci. 2000 Aug 1;20(15):5775-81. doi: 10.1523/JNEUROSCI.20-15-05775.2000. J Neurosci. 2000. PMID: 10908618 Free PMC article.
-
GSK-3β inhibitor TDZD-8 reduces neonatal hypoxic-ischemic brain injury in mice.CNS Neurosci Ther. 2017 May;23(5):405-415. doi: 10.1111/cns.12683. Epub 2017 Mar 2. CNS Neurosci Ther. 2017. PMID: 28256059 Free PMC article.
-
Subtoxic N-methyl-D-aspartate delayed neuronal death in ischemic brain injury through TrkB receptor- and calmodulin-mediated PI-3K/Akt pathway activation.Hippocampus. 2007;17(7):525-37. doi: 10.1002/hipo.20289. Hippocampus. 2007. PMID: 17492691
-
FGF21 promotes functional recovery after hypoxic-ischemic brain injury in neonatal rats by activating the PI3K/Akt signaling pathway via FGFR1/β-klotho.Exp Neurol. 2019 Jul;317:34-50. doi: 10.1016/j.expneurol.2019.02.013. Epub 2019 Feb 23. Exp Neurol. 2019. PMID: 30802446
Cited by
-
Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression.Psychopharmacology (Berl). 2018 Aug;235(8):2195-2220. doi: 10.1007/s00213-018-4950-4. Epub 2018 Jun 30. Psychopharmacology (Berl). 2018. PMID: 29961124 Free PMC article. Review.
-
Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges.Front Cell Neurosci. 2017 May 8;11:78. doi: 10.3389/fncel.2017.00078. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28533743 Free PMC article. Review.
-
Are TrkB receptor agonists the right tool to fulfill the promises for a therapeutic value of the brain-derived neurotrophic factor?Neural Regen Res. 2024 Jan;19(1):29-34. doi: 10.4103/1673-5374.374138. Neural Regen Res. 2024. PMID: 37488840 Free PMC article. Review.
-
Neuroimaging Genomics a Predictor of Major Depressive Disorder (MDD).Mol Neurobiol. 2024 Jun;61(6):3427-3440. doi: 10.1007/s12035-023-03775-0. Epub 2023 Nov 22. Mol Neurobiol. 2024. PMID: 37989980 Review.
-
Neonatal inhibition of Na+-K+-2Cl--cotransporter prevents ketamine induced spatial learning and memory impairments.Neurotoxicol Teratol. 2017 Mar-Apr;60:82-86. doi: 10.1016/j.ntt.2016.11.001. Epub 2016 Nov 5. Neurotoxicol Teratol. 2017. PMID: 27826117 Free PMC article.
References
-
- Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79: 1431–1568. - PubMed
-
- Ezquer ME, Valdez SR, Seltzer AM (2006) Inflammatory responses of the substantia nigra after acute hypoxia in neonatal rats. Exp Neurol 197: 391–398. - PubMed
-
- Han BH, D'Costa A, Back SA, Parsadanian M, Patel S, et al. (2000) BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia. Neurobiol Dis 7: 38–53. - PubMed
-
- Cho S, Liu D, Gonzales C, Zaleska MM, Wood A (2003) Temporal assessment of caspase activation in experimental models of focal and global ischemia. Brain Res 982: 146–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

