GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza
- PMID: 24551202
- PMCID: PMC3925208
- DOI: 10.1371/journal.pone.0088979
GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza
Abstract
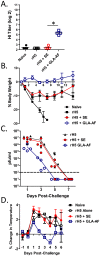
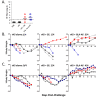
The ongoing threat from Influenza necessitates the development of new vaccine and adjuvant technologies that can maximize vaccine immunogenicity, shorten production cycles, and increase global vaccine supply. Currently, the most successful adjuvants for Influenza vaccines are squalene-based oil-in-water emulsions. These adjuvants enhance seroprotective antibody titers to homologous and heterologous strains of virus, and augment a significant dose sparing activity that could improve vaccine manufacturing capacity. As an alternative to an emulsion, we tested a simple lipid-based aqueous formulation containing a synthetic TLR4 ligand (GLA-AF) for its ability to enhance protection against H5N1 infection. GLA-AF was very effective in adjuvanting recombinant H5 hemagglutinin antigen (rH5) in mice and was as potent as the stable emulsion, SE. Both adjuvants induced similar antibody titers using a sub-microgram dose of rH5, and both conferred complete protection against a highly pathogenic H5N1 challenge. However, GLA-AF was the superior adjuvant in ferrets. GLA-AF stimulated a broader antibody response than SE after both the prime and boost immunization with rH5, and ferrets were better protected against homologous and heterologous strains of H5N1 virus. Thus, GLA-AF is a potent emulsion-free adjuvant that warrants consideration for pandemic influenza vaccine development.
Conflict of interest statement
Figures



Similar articles
-
Recombinant H5 hemagglutinin adjuvanted with nanoemulsion protects ferrets against pathogenic avian influenza virus challenge.Vaccine. 2019 Mar 14;37(12):1591-1600. doi: 10.1016/j.vaccine.2019.02.002. Epub 2019 Feb 19. Vaccine. 2019. PMID: 30795941
-
A nanoemulsion-adjuvanted intranasal H5N1 influenza vaccine protects ferrets against homologous and heterologous H5N1 lethal challenge.Vaccine. 2019 Sep 30;37(42):6162-6170. doi: 10.1016/j.vaccine.2019.08.071. Epub 2019 Sep 5. Vaccine. 2019. PMID: 31495593
-
Adjuvant solution for pandemic influenza vaccine production.Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17585-90. doi: 10.1073/pnas.1207308109. Epub 2012 Oct 8. Proc Natl Acad Sci U S A. 2012. PMID: 23045649 Free PMC article.
-
Prepandemic influenza vaccine H5N1 (split virion, inactivated, adjuvanted) [Prepandrix]: a review of its use as an active immunization against influenza A subtype H5N1 virus.BioDrugs. 2008;22(5):279-92. doi: 10.2165/00063030-200822050-00001. BioDrugs. 2008. PMID: 18778110 Review.
-
New strategies for the development of H5N1 subtype influenza vaccines: progress and challenges.BioDrugs. 2011 Oct 1;25(5):285-98. doi: 10.1007/BF03256169. BioDrugs. 2011. PMID: 21942913 Review.
Cited by
-
Ferret models of viral pathogenesis.Virology. 2015 May;479-480:259-70. doi: 10.1016/j.virol.2015.03.017. Epub 2015 Mar 26. Virology. 2015. PMID: 25816764 Free PMC article. Review.
-
Novel Anti-Nicotine Vaccine Using a Trimeric Coiled-Coil Hapten Carrier.PLoS One. 2014 Dec 10;9(12):e114366. doi: 10.1371/journal.pone.0114366. eCollection 2014. PLoS One. 2014. PMID: 25494044 Free PMC article.
-
Next-generation influenza vaccines: opportunities and challenges.Nat Rev Drug Discov. 2020 Apr;19(4):239-252. doi: 10.1038/s41573-019-0056-x. Epub 2020 Feb 14. Nat Rev Drug Discov. 2020. PMID: 32060419 Free PMC article. Review.
-
Improved Immune Responses in Young and Aged Mice with Adjuvanted Vaccines against H1N1 Influenza Infection.Front Immunol. 2018 Feb 19;9:295. doi: 10.3389/fimmu.2018.00295. eCollection 2018. Front Immunol. 2018. PMID: 29515589 Free PMC article.
-
A Formulated TLR7/8 Agonist is a Flexible, Highly Potent and Effective Adjuvant for Pandemic Influenza Vaccines.Sci Rep. 2017 Apr 21;7:46426. doi: 10.1038/srep46426. Sci Rep. 2017. PMID: 28429728 Free PMC article.
References
-
- World Health Organization website. Available: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_tabl.... Accessed 2014 Jan 23.
-
- Lam TT, Wang J, Shen Y, Zhou B, Duan L, et al.. (2013) The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. doi: 10.1038/nature12515. - DOI - PMC - PubMed
-
- Zhang Q, Shi J, Deng G, Guo J, Zeng X, et al. (2013) H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science 341: 410–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

