Photo-activated psoralen binds the ErbB2 catalytic kinase domain, blocking ErbB2 signaling and triggering tumor cell apoptosis
- PMID: 24551203
- PMCID: PMC3925176
- DOI: 10.1371/journal.pone.0088983
Photo-activated psoralen binds the ErbB2 catalytic kinase domain, blocking ErbB2 signaling and triggering tumor cell apoptosis
Abstract
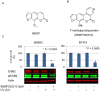
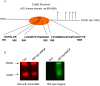
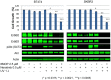
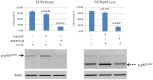
Photo-activation of psoralen with UVA irradiation, referred to as PUVA, is used in the treatment of proliferative skin disorders. The anti-proliferative effects of PUVA have been largely attributed to psoralen intercalation of DNA, which upon UV treatment, triggers the formation of interstrand DNA crosslinks (ICL) that inhibit transcription and DNA replication. Here, we show that PUVA exerts antitumor effects in models of human breast cancer that overexpress the ErbB2 receptor tyrosine kinase oncogene, through a new mechanism. Independent of ICL formation, the antitumor effects of PUVA in ErbB2+ breast cancer models can instead be mediated through inhibition of ErbB2 activation and signaling. Using a mass spectroscopy-based approach, we show for the first time that photo-activated 8MOP (8-methoxypsoralen) interacts with the ErbB2 catalytic autokinase domain. Furthermore, PUVA can reverse therapeutic resistance to lapatinib and other ErbB2 targeted therapies, including resistance mediated via expression of a phosphorylated, truncated form of ErbB2 (p85(ErbB2)) that is preferentially expressed in tumor cell nuclei. Current ErbB2 targeted therapies, small molecule kinase inhibitors or antibodies, do not block the phosphorylated, activated state of p85(ErbB2). Here we show that PUVA reduced p85(ErbB2) phosphorylation leading to tumor cell apoptosis. Thus, in addition to its effects on DNA and the formation of ICL, PUVA represents a novel ErbB2 targeted therapy for the treatment of ErbB2+ breast cancers, including those that have developed resistance to other ErbB2 targeted therapies.
Conflict of interest statement
Figures



References
-
- Hearst JE, Isaacs ST, Kanne D, Rapoport H, Straub K (1984) The reaction of the psoralens with deoxyribonucleic acid. Q Rev Biophys 17: 1–44. - PubMed
-
- Cimino GD, Gamper HB, Isaacs ST, Hearst JE (1985) Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem 54: 1151–1193. - PubMed
-
- Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA (1974) Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med 291: 1207–1211. - PubMed
-
- Berger CL, Cantor C, Welsh J, Dervan P, Begley T, et al. (1985) Comparison of synthetic psoralen derivatives and 8-MOP in the inhibition of lymphocyte proliferation. Ann N Y Acad Sci 453: 80–90. - PubMed
-
- Edelson RL, Berger C, Gasparro F, Jegasothy B, Heald P, et al. (1987) Treatment of cutaneous Tcell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med 316: 297–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

