Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation
- PMID: 24551211
- PMCID: PMC3923876
- DOI: 10.1371/journal.pone.0089025
Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation
Expression of concern in
-
Expression of Concern: Sox9 Potentiates BMP2-Induced Chondrogenic Differentiation and Inhibits BMP2-Induced Osteogenic Differentiation.PLoS One. 2021 Apr 1;16(4):e0249684. doi: 10.1371/journal.pone.0249684. eCollection 2021. PLoS One. 2021. PMID: 33793677 Free PMC article. No abstract available.
Abstract
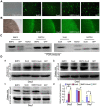
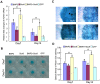
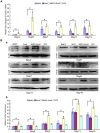
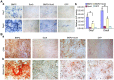
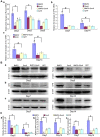
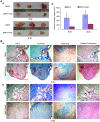
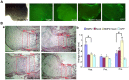
Bone morphogenetic protein 2 (BMP2) is one of the key chondrogenic growth factors involved in the cartilage regeneration. However, it also exhibits osteogenic abilities and triggers endochondral ossification. Effective chondrogenesis and inhibition of BMP2-induced osteogenesis and endochondral ossification can be achieved by directing the mesenchymal stem cells (MSCs) towards chondrocyte lineage with chodrogenic factors, such as Sox9. Here we investigated the effects of Sox9 on BMP2-induced chondrogenic and osteogenic differentiation of MSCs. We found exogenous overexpression of Sox9 enhanced the BMP2-induced chondrogenic differentiation of MSCs in vitro. Also, it inhibited early and late osteogenic differentiation of MSCs in vitro. Subcutaneous stem cell implantation demonstrated Sox9 potentiated BMP2-induced cartilage formation and inhibited endochondral ossification. Mouse limb cultures indicated that BMP2 and Sox9 acted synergistically to stimulate chondrocytes proliferation, and Sox9 inhibited BMP2-induced chondrocytes hypertrophy and ossification. This study strongly suggests that Sox9 potentiates BMP2-induced MSCs chondrogenic differentiation and cartilage formation, and inhibits BMP2-induced MSCs osteogenic differentiation and endochondral ossification. Thus, exogenous overexpression of Sox9 in BMP2-induced mesenchymal stem cells differentiation may be a new strategy for cartilage tissue engineering.
Conflict of interest statement
Figures
References
-
- Benthien JP, Schwaninger M, Behrens P (2011) We do not have evidence based methods for the treatment of cartilage defects in the knee. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 19: 543–552. - PubMed
-
- Rodriguez-Merchan EC (2012) Regeneration of articular cartilage of the knee. Rheumatology international. - PubMed
-
- Kao YJ, Ho J, Allen CR (2011) Evaluation and management of osteochondral lesions of the knee. The Physician and sportsmedicine 39: 60–69. - PubMed
-
- Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, et al. (2009) Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 27: 1432–1438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

