A GmRAV ortholog is involved in photoperiod and sucrose control of flowering time in soybean
- PMID: 24551235
- PMCID: PMC3925180
- DOI: 10.1371/journal.pone.0089145
A GmRAV ortholog is involved in photoperiod and sucrose control of flowering time in soybean
Abstract
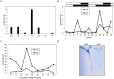
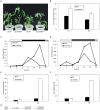
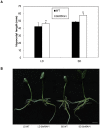
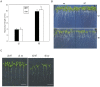
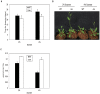
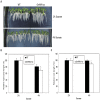
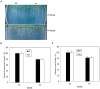
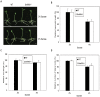
Photoperiod and sucrose levels play a key role in the control of flowering. GmRAV reflected a diurnal rhythm with the highest expression at 4 h after the beginning of a dark period in soybean leaves, and was highly up-regulated under short-day (SD) conditions, despite of not following a diurnal pattern under long-day (LD) conditions. GmRAV-i (GmRAV-inhibition) transgenic soybean exhibited early flowering phenotype. Two of the FT Arabidopsis homologs, GmFT2a and GmFT5a, were highly expressed in the leaves of soybeans with inhibition (-i) of GmRAV under SD conditions. Moreover, the transcript levels of the two FT homologs in GmRAV-i soybeans were more sensitive to SD conditions than LD conditions compared to the WT plant. GmRAV-i soybeans and Arabidopsis rav mutants showed more sensitive hypocotyl elongation responses when compared with wild-type seedlings, and GmRAV-ox overevpressed in tobacco revealed no sensitive changes in hypocotyl length. These indicated that GmRAV was a novel negative regulator of SD-mediated flowering and hypocotyl elongation. Although sucrose has been suggested to promote flowering induction in many plant species, high concentration of sucrose (4% [w/v]) applied into media defer flowering time in Arabidopsis wild-type and rav mutant. This delayed flowering stage might be caused by reduction of LEAFY expression. Furthermore, Arabidopsis rav mutants and GmRAV-i soybean plants were less sensitive to sucrose by the inhibition assays of hypocotyls and roots growth. In contrast, transgenic GmRAV overexpressing (-ox) tobacco plants displayed more sensitivity to sucrose. In conclusion, GmRAV was inferred to have a fundamental function in photoperiod, darkness, and sucrose signaling responses to regulate plant development and flowering induction.
Conflict of interest statement
Figures
Similar articles
-
Roles for a soybean RAV-like orthologue in shoot regeneration and photoperiodicity inferred from transgenic plants.J Exp Bot. 2012 May;63(8):3257-70. doi: 10.1093/jxb/ers056. Epub 2012 Mar 2. J Exp Bot. 2012. PMID: 22389516
-
Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean.Plant Physiol. 2010 Nov;154(3):1220-31. doi: 10.1104/pp.110.160796. Epub 2010 Sep 23. Plant Physiol. 2010. PMID: 20864544 Free PMC article.
-
GmFT2a, a soybean homolog of FLOWERING LOCUS T, is involved in flowering transition and maintenance.PLoS One. 2011;6(12):e29238. doi: 10.1371/journal.pone.0029238. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22195028 Free PMC article.
-
Molecular Genetic Understanding of Photoperiodic Regulation of Flowering Time in Arabidopsis and Soybean.Int J Mol Sci. 2021 Dec 31;23(1):466. doi: 10.3390/ijms23010466. Int J Mol Sci. 2021. PMID: 35008892 Free PMC article. Review.
-
Molecular mechanisms for the photoperiodic regulation of flowering in soybean.J Integr Plant Biol. 2021 Jun;63(6):981-994. doi: 10.1111/jipb.13021. Epub 2021 Apr 26. J Integr Plant Biol. 2021. PMID: 33090664 Review.
Cited by
-
Comprehensive analysis of cucumber RAV family genes and functional characterization of CsRAV1 in salt and ABA tolerance in cucumber.Front Plant Sci. 2023 Feb 2;14:1115874. doi: 10.3389/fpls.2023.1115874. eCollection 2023. Front Plant Sci. 2023. PMID: 36818828 Free PMC article.
-
NtRAV4 negatively regulates drought tolerance in Nicotiana tabacum by enhancing antioxidant capacity and defence system.Plant Cell Rep. 2022 Aug;41(8):1775-1788. doi: 10.1007/s00299-022-02896-5. Epub 2022 Jul 5. Plant Cell Rep. 2022. PMID: 35789421
-
Genome-Wide Analysis of the RAV Family in Soybean and Functional Identification of GmRAV-03 Involvement in Salt and Drought Stresses and Exogenous ABA Treatment.Front Plant Sci. 2017 Jun 6;8:905. doi: 10.3389/fpls.2017.00905. eCollection 2017. Front Plant Sci. 2017. PMID: 28634481 Free PMC article.
-
Expression of Three Related to ABI3/VP1 Genes in Medicago truncatula Caused Increased Stress Resistance and Branch Increase in Arabidopsis thaliana.Front Plant Sci. 2020 May 25;11:611. doi: 10.3389/fpls.2020.00611. eCollection 2020. Front Plant Sci. 2020. PMID: 32523590 Free PMC article.
-
GmRAV confers ecological adaptation through photoperiod control of flowering time and maturity in soybean.Plant Physiol. 2021 Sep 4;187(1):361-377. doi: 10.1093/plphys/kiab255. Plant Physiol. 2021. PMID: 34618136 Free PMC article.
References
-
- Coupland G (1995) Genetic and environmental control of flowering time in Arabidopsis. Trends Genet 11: 393–397. - PubMed
-
- Amasino RM (1996) Control of flowering time in plants. Curr Opin Genet Devel 6: 480–487. - PubMed
-
- Koornneef M, Alonso-Blanco C, Peeters AJM, Soppe W (1998) Genetic control of flowering time in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol Biol 49: 345–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

