Filamin B regulates chondrocyte proliferation and differentiation through Cdk1 signaling
- PMID: 24551245
- PMCID: PMC3925234
- DOI: 10.1371/journal.pone.0089352
Filamin B regulates chondrocyte proliferation and differentiation through Cdk1 signaling
Abstract
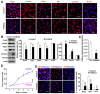
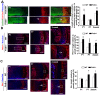
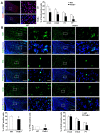
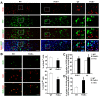
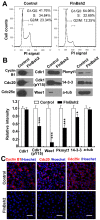
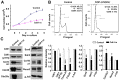
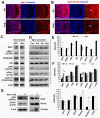
Humans who harbor loss of function mutations in the actin-associated filamin B (FLNB) gene develop spondylocarpotarsal syndrome (SCT), a disorder characterized by dwarfism (delayed bone formation) and premature fusion of the vertebral, carpal and tarsal bones (premature differentiation). To better understand the cellular and molecular mechanisms governing these seemingly divergent processes, we generated and characterized FlnB knockdown ATDC5 cell lines. We found that FlnB knockdown led to reduced proliferation and enhanced differentiation in chondrocytes. Within the shortened growth plate of postnatal FlnB(-/-) mice long bone, we observed a similarly progressive decline in the number of rapidly proliferating chondrocytes and premature differentiation characterized by an enlarged prehypertrophic zone, a widened Col2a1(+)/Col10a1(+) overlapping region, but relatively reduced hypertrophic zone length. The reduced chondrocyte proliferation and premature differentiation were, in part, attributable to enhanced G2/M phase progression, where fewer FlnB deficient ATDC5 chondrocytes resided in the G2/M phase of the cell cycle. FlnB loss reduced Cdk1 phosphorylation (an inhibitor of G2/M phase progression) and Cdk1 inhibition in chondrocytes mimicked the null FlnB, premature differentiation phenotype, through a β1-integrin receptor- Pi3k/Akt (a key regulator of chondrocyte differentiation) mediated pathway. In this context, the early prehypertrophic differentiation provides an explanation for the premature differentiation seen in this disorder, whereas the progressive decline in proliferating chondrocytes would ultimately lead to reduced chondrocyte production and shortened bone length. These findings begin to define a role for filamin proteins in directing both cell proliferation and differentiation through indirect regulation of cell cycle associated proteins.
Conflict of interest statement
Figures
Similar articles
-
Formin 1 and filamin B physically interact to coordinate chondrocyte proliferation and differentiation in the growth plate.Hum Mol Genet. 2014 Sep 1;23(17):4663-73. doi: 10.1093/hmg/ddu186. Epub 2014 Apr 23. Hum Mol Genet. 2014. PMID: 24760772 Free PMC article.
-
Filamin B mutations cause chondrocyte defects in skeletal development.Hum Mol Genet. 2007 Jul 15;16(14):1661-75. doi: 10.1093/hmg/ddm114. Epub 2007 May 17. Hum Mol Genet. 2007. PMID: 17510210
-
The Indispensable Role of Cyclin-Dependent Kinase 1 in Skeletal Development.Sci Rep. 2016 Feb 10;6:20622. doi: 10.1038/srep20622. Sci Rep. 2016. PMID: 26860366 Free PMC article.
-
Filamin B: The next hotspot in skeletal research?J Genet Genomics. 2017 Jul 20;44(7):335-342. doi: 10.1016/j.jgg.2017.04.007. Epub 2017 Jul 6. J Genet Genomics. 2017. PMID: 28739045 Review.
-
The filamin-B-refilin axis - spatiotemporal regulators of the actin-cytoskeleton in development and disease.J Cell Sci. 2018 Apr 13;131(8):jcs213959. doi: 10.1242/jcs.213959. J Cell Sci. 2018. PMID: 29654161 Review.
Cited by
-
Mechanisms and Effects on HBV Replication of the Interaction between HBV Core Protein and Cellular Filamin B.Virol Sin. 2018 Apr;33(2):162-172. doi: 10.1007/s12250-018-0023-4. Epub 2018 Mar 28. Virol Sin. 2018. PMID: 29594956 Free PMC article.
-
Chronic exposure to chewing tobacco selects for overexpression of stearoyl-CoA desaturase in normal oral keratinocytes.Cancer Biol Ther. 2015;16(11):1593-603. doi: 10.1080/15384047.2015.1078022. Epub 2015 Sep 21. Cancer Biol Ther. 2015. PMID: 26391970 Free PMC article.
-
Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli.Front Cell Dev Biol. 2020 Sep 22;8:555378. doi: 10.3389/fcell.2020.555378. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33072743 Free PMC article.
-
Formin 1 and filamin B physically interact to coordinate chondrocyte proliferation and differentiation in the growth plate.Hum Mol Genet. 2014 Sep 1;23(17):4663-73. doi: 10.1093/hmg/ddu186. Epub 2014 Apr 23. Hum Mol Genet. 2014. PMID: 24760772 Free PMC article.
-
MTMR7 regulates human spermatogonial stem cells proliferation and migration via targeting FLNB.PLoS One. 2025 Jul 10;20(7):e0327669. doi: 10.1371/journal.pone.0327669. eCollection 2025. PLoS One. 2025. PMID: 40638605 Free PMC article.
References
-
- Farrington-Rock C, Firestein MH, Bicknell LS, Superti-Furga A, Bacino CA, et al. (2006) Mutations in two regions of FLNB result in atelosteogenesis I and III. Hum Mutat 27: 705–710. - PubMed
-
- Krakow D, Robertson SP, King LM, Morgan T, Sebald ET, et al. (2004) Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat Genet 36: 405–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

