Alveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53
- PMID: 24551275
- PMCID: PMC3925899
Alveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53
Abstract
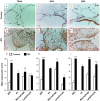
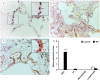
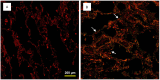
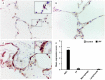
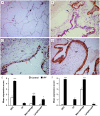
Idiopathic pulmonary fibrosis (IPF) is a progressive, debilitating, and fatal lung disease of unknown aetiology with no current cure. The pathogenesis of IPF remains unclear but repeated alveolar epithelial cell (AEC) injuries and subsequent apoptosis are believed to be among the initiating/ongoing triggers. However, the precise mechanism of apoptotic induction is hitherto elusive. In this study, we investigated expression of a panel of pro-apoptotic and cell cycle regulatory proteins in 21 IPF and 19 control lung tissue samples. We reveal significant upregulation of the apoptosis-inducing ligand TRAIL and its cognate receptors DR4 and DR5 in AEC within active lesions of IPF lungs. This upregulation was accompanied by pro-apoptotic protein p53 overexpression. In contrast, myofibroblasts within the fibroblastic foci of IPF lungs exhibited high TRAIL, DR4 and DR5 expression but negligible p53 expression. Similarly, p53 expression was absent or negligible in IPF and control alveolar macrophages and lymphocytes. No significant differences in TRAIL expression were noted in these cell types between IPF and control lungs. However, DR4 and DR5 upregulation was detected in IPF alveolar macrophages and lymphocytes. The marker of cellular senescence p21(WAF1) was upregulated within affected AEC in IPF lungs. Cell cycle regulatory proteins Cyclin D1 and SOCS3 were significantly enhanced in AEC within the remodelled fibrotic areas of IPF lungs but expression was negligible in myofibroblasts. Taken together these findings suggest that, within the remodelled fibrotic areas of IPF, AEC can display markers associated with proliferation, senescence, and apoptotosis, where TRAIL could drive the apoptotic response. Clear understanding of disease processes and identification of therapeutic targets will direct us to develop effective therapies for IPF.
Keywords: DR4; DR5; Idiopathic pulmonary fibrosis; TRAIL; immunohistochemistry; p21WAF1; p53.
Figures
References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. - PMC - PubMed
-
- Dacic S, Yousem SA. Histologic classification of idiopathic chronic interstitial pneumonias. Am J Respir Cell Mol Biol. 2003;29:S5–S9. - PubMed
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and mechanisms? Eur Respir J. 2007;30:835–839. - PubMed
-
- Selman M, King TE, Pardo A. American Thoracic Society, European Respiratory Society & American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
