G-protein coupled receptor-evoked glutamate exocytosis from astrocytes: role of prostaglandins
- PMID: 24551459
- PMCID: PMC3914554
- DOI: 10.1155/2014/254574
G-protein coupled receptor-evoked glutamate exocytosis from astrocytes: role of prostaglandins
Abstract
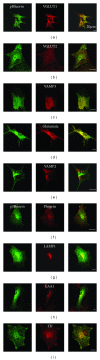
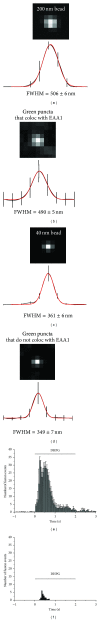
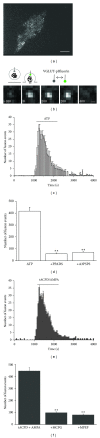
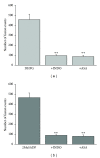
Astrocytes are highly secretory cells, participating in rapid brain communication by releasing glutamate. Recent evidences have suggested that this process is largely mediated by Ca(2+)-dependent regulated exocytosis of VGLUT-positive vesicles. Here by taking advantage of VGLUT1-pHluorin and TIRF illumination, we characterized mechanisms of glutamate exocytosis evoked by endogenous transmitters (glutamate and ATP), which are known to stimulate Ca(2+) elevations in astrocytes. At first we characterized the VGLUT1-pHluorin expressing vesicles and found that VGLUT1-positive vesicles were a specific population of small synaptic-like microvesicles containing glutamate but which do not express VGLUT2. Endogenous mediators evoked a burst of exocytosis through activation of G-protein coupled receptors. Subsequent glutamate exocytosis was reduced by about 80% upon pharmacological blockade of the prostaglandin-forming enzyme, cyclooxygenase. On the other hand, receptor stimulation was accompanied by extracellular release of prostaglandin E2 (PGE2). Interestingly, administration of exogenous PGE2 produced per se rapid, store-dependent burst exocytosis of glutamatergic vesicles in astrocytes. Finally, when PGE2-neutralizing antibody was added to cell medium, transmitter-evoked exocytosis was again significantly reduced (by about 50%). Overall these data indicate that cyclooxygenase products are responsible for a major component of glutamate exocytosis in astrocytes and that large part of such component is sustained by autocrine/paracrine action of PGE2.
Figures
Similar articles
-
Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes.J Physiol. 2003 Dec 1;553(Pt 2):407-14. doi: 10.1113/jphysiol.2003.046706. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500777 Free PMC article.
-
Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate.Nat Neurosci. 2004 Jun;7(6):613-20. doi: 10.1038/nn1246. Epub 2004 May 23. Nat Neurosci. 2004. PMID: 15156145
-
SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy.J Neuroimmunol. 2008 Jul 31;198(1-2):82-91. doi: 10.1016/j.jneuroim.2008.04.015. Epub 2008 Jun 6. J Neuroimmunol. 2008. PMID: 18538866
-
Morphological evidence for vesicular glutamate release from astrocytes.Neuroscience. 2009 Jan 12;158(1):260-5. doi: 10.1016/j.neuroscience.2008.03.074. Epub 2008 Apr 9. Neuroscience. 2009. PMID: 18479831 Review.
-
CXCR4-mediated glutamate exocytosis from astrocytes.J Neuroimmunol. 2010 Jul 27;224(1-2):13-21. doi: 10.1016/j.jneuroim.2010.05.004. Epub 2010 Jun 30. J Neuroimmunol. 2010. PMID: 20580441 Review.
Cited by
-
Microglia at the Tripartite Synapse during Postnatal Development: Implications for Autism Spectrum Disorders and Schizophrenia.Cells. 2023 Dec 13;12(24):2827. doi: 10.3390/cells12242827. Cells. 2023. PMID: 38132147 Free PMC article. Review.
-
Metabolic dynamics in astrocytes and microglia during post-natal development and their implications for autism spectrum disorders.Front Cell Neurosci. 2024 Feb 14;18:1354259. doi: 10.3389/fncel.2024.1354259. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38419654 Free PMC article. Review.
-
Brain oxidative stress mediates anxiety-like behavior induced by indomethacin in zebrafish: protective effect of alpha-tocopherol.Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1715-1725. doi: 10.1007/s00210-023-02661-9. Epub 2023 Sep 18. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37721555 Free PMC article.
-
A computational study of astrocytic glutamate influence on post-synaptic neuronal excitability.PLoS Comput Biol. 2018 Apr 16;14(4):e1006040. doi: 10.1371/journal.pcbi.1006040. eCollection 2018 Apr. PLoS Comput Biol. 2018. PMID: 29659572 Free PMC article.
-
Astrocyte Dysfunctions in Obsessive Compulsive Disorder: Rethinking Neurobiology and Therapeutic Targets.J Neurochem. 2025 May;169(5):e70092. doi: 10.1111/jnc.70092. J Neurochem. 2025. PMID: 40400176 Free PMC article. Review.
References
-
- Bezzi P, Carmignoto G, Pasti L, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391(6664):281–285. - PubMed
-
- Santello M, Bezzi P, Volterra A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69(5):988–1001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

