Development of Stereocontrolled Palladium(II)-Catalyzed Domino Heck/Suzuki β,α-Diarylation Reactions with Chelating Vinyl Ethers and Arylboronic Acids
- PMID: 24551492
- PMCID: PMC3922440
- DOI: 10.1002/open.201100010
Development of Stereocontrolled Palladium(II)-Catalyzed Domino Heck/Suzuki β,α-Diarylation Reactions with Chelating Vinyl Ethers and Arylboronic Acids
Abstract
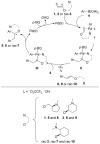
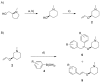
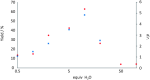
A stereoselective and 1,4-benzoquinone-mediated palladium(II)-catalyzed Heck/Suzuki domino reaction involving metal coordinating cyclic methylamino vinyl ethers and a number of electronically diverse arylboronic acids has been developed and studied. Diastereomeric ratios up to 39:1 and 78 % isolated yields were obtained. The stereoselectivity of the reaction was found to be highly dependent on the nature of the arylboronic acid and the amount of water present in the reaction mixture. Thus, a domino β,α-diarylation-reduction of chelating vinyl ethers can now be accomplished and stereochemically controlled, given that optimized conditions and an appropriate chiral auxiliary are used. To the best of our knowledge, this represents the first example of a stereoselective, oxidative Heck/Suzuki domino reaction in the literature.
Keywords: chirality; domino reactions; palladium; stereoselective catalysis; water effects.
Figures


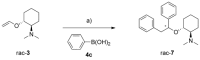
References
-
- Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. <. - PubMed
-
- de Meijere A, Stang PJ, editors. Metal-Catalyzed Cross-Coupling Reactions. 2. Wiley-VCH; 2004.
-
- Oestreich M, editor. The Mizoroki–Heck Reaction. Chichester: John Wiley & Sons; 2009.
-
- Heck RF. J. Am. Chem. Soc. 1971;93:6896.
-
- Heck RF. Fortschr. Chem. Forsch. 1971;16:221.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

