Screening of N-alkyl-cyanoacetamido oximes as substitutes for N-hydroxysuccinimide
- PMID: 24551503
- PMCID: PMC3922453
- DOI: 10.1002/open.201200012
Screening of N-alkyl-cyanoacetamido oximes as substitutes for N-hydroxysuccinimide
Abstract
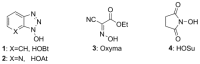
Peptide-bond formation is a pivotal process in the synthesis of peptide oligomers. Among the various coupling methodologies described, carbodiimides combine strong acylation potency and smooth reaction conditions, and they are commonly used in the presence of N-hydroxylamine additives. In recent years, acidic oxime templates, mainly ethyl 2-cyano-2-(hydroxyimino) acetate (Oxyma), have emerged as highly reactive alternatives to the classic and explosive-prone benzotriazolic additives, 1-hydroxybenzotriazole (HOBt) and 1-hydroxy-7-azabenzotriazole (HOAt). However, to achieve certain biochemical targets, less reactive species, such as N-hydroxysuccinimide (HOSu) esters, are often required to obtain stability under aqueous conditions. In the present study, we report on a new family of water-soluble N-alkyl-cyanoacetamido oximes, most of which have proven useful in the construction of active carbonates for the introduction of fluorenylmethoxycarbonyl (Fmoc) with minimal impact of dipeptide impurities. We performed a direct comparison of these new N-alkyl-cyanoacetamido oximes with HOSu in order to evaluate their capacity to retain optical purity and their coupling efficiency in the assembly of bulky residues.
Keywords: N-hydroxysuccinimides; additives; oximes; peptide syntheses; peptide-bond formations.
Figures

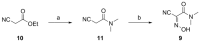
Similar articles
-
Oxyma-B, an excellent racemization suppressor for peptide synthesis.Org Biomol Chem. 2014 Nov 14;12(42):8379-85. doi: 10.1039/c4ob01612b. Org Biomol Chem. 2014. PMID: 25233797
-
Sulfonate esters of 1-hydroxypyridin-2(1H)-one and ethyl 2-cyano-2-(hydroxyimino)acetate (oxyma) as effective peptide coupling reagents to replace 1-hydroxybenzotriazole and 1-hydroxy-7-azabenzotriazole.Chem Pharm Bull (Tokyo). 2010 Apr;58(4):501-6. doi: 10.1248/cpb.58.501. Chem Pharm Bull (Tokyo). 2010. PMID: 20410632
-
Reactions of monoaryl-substituted methylenecyclobutanes and methylenecyclopropanes with 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt), and 1-hydroxysuccinimide (HOSu).J Org Chem. 2009 Mar 20;74(6):2516-20. doi: 10.1021/jo9000299. J Org Chem. 2009. PMID: 19222218
-
The development of new oximes and the evaluation of their reactivating, therapeutic and neuroprotective efficacy against tabun.Mini Rev Med Chem. 2008 Oct;8(11):1134-43. doi: 10.2174/138955708785909871. Mini Rev Med Chem. 2008. PMID: 18855728 Review.
-
Steroidal Oximes: Useful Compounds with Antitumor Activities.Curr Med Chem. 2018 Feb 21;25(6):660-686. doi: 10.2174/0929867324666171003115400. Curr Med Chem. 2018. PMID: 28971759 Review.
Cited by
-
Understanding OxymaPure as a Peptide Coupling Additive: A Guide to New Oxyma Derivatives.ACS Omega. 2022 Feb 9;7(7):6007-6023. doi: 10.1021/acsomega.1c06342. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224362 Free PMC article.
-
OxymaPure/DIC: an efficient reagent for the synthesis of a novel series of 4-[2-(2-acetylaminophenyl)-2-oxo-acetylamino] benzoyl amino acid ester derivatives.Molecules. 2013 Nov 28;18(12):14747-59. doi: 10.3390/molecules181214747. Molecules. 2013. PMID: 24288002 Free PMC article.
References
-
- El-Faham A, Albericio F. Chem. Rev. 2011;111:6557–6602. - PubMed
-
- Valeur E, Bradley M. Chem. Soc. Rev. 2009;38:606–631. - PubMed
-
- Han S-Y, Kim Y-A. Tetrahedron. 2004;60:2447–2467.
-
- Humphrey JM, Chamberlin AR. Chem. Rev. 1997;97:2243–2266. - PubMed
-
- Weisz I, Roboz J, Bekesi G. Tetrahedron Lett. 1996;37:563–566.
LinkOut - more resources
Full Text Sources

