Anti-invasive effects of Celastrus Orbiculatus extract on interleukin-1 beta and tumour necrosis factor-alpha combination-stimulated fibroblast-like synoviocytes
- PMID: 24552146
- PMCID: PMC3938040
- DOI: 10.1186/1472-6882-14-62
Anti-invasive effects of Celastrus Orbiculatus extract on interleukin-1 beta and tumour necrosis factor-alpha combination-stimulated fibroblast-like synoviocytes
Abstract
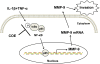
Background: Invasion of fibroblast-like synoviocytes (FLSs) is critical in the pathogenesis of rheumatoid arthritis (RA). The metalloproteinases (MMPs) and activator of nuclear factor-kappa B (NF-κB) pathway play a critical role in RA-FLS invasion induced by interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α). The present study aimed to explore the anti-invasive activity and mechanism of Celastrus orbiculatus extract (COE) on IL-1β and TNF-α combination-stimulated human RA-FLSs.
Methods: We investigated the effect of COE on IL-1β and TNF-α combination-induced FLS invasion as well as MMP expression and explored upstream signal transduction.
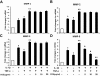
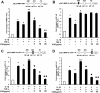
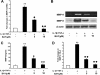
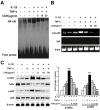
Results: COE suppressed IL-1β and TNF-α combination-stimulated FLSs invasion by inhibiting MMP-9 expression and activity. Furthermore, our results revealed that COE inhibited the transcriptional activity of MMP-9 by suppression of the binding activity of NF-κB in the MMP-9 promoter, and inhibited IκBα phosphorylation and nuclear translocation of NF-κB.
Conclusions: COE inhibits IL-1β and TNF-α combination-induced FLSs invasion by suppressing NF-κB-mediated MMP-9 expression.
Figures


Similar articles
-
Gastrodia elata attenuates inflammatory response by inhibiting the NF-κB pathway in rheumatoid arthritis fibroblast-like synoviocytes.Biomed Pharmacother. 2017 Jan;85:177-181. doi: 10.1016/j.biopha.2016.11.136. Epub 2016 Dec 6. Biomed Pharmacother. 2017. PMID: 27936399
-
Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes.Eur J Pharmacol. 2009 Jun 24;613(1-3):167-75. doi: 10.1016/j.ejphar.2009.04.026. Epub 2009 Apr 18. Eur J Pharmacol. 2009. PMID: 19379726
-
[Mechanism of 4-methylcatechol in inhibiting fibroblast-like synoviocyte migration and suppressing inflammatory responses in treatment of rheumatoid arthritis].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025 Aug 15;39(8):1051-1060. doi: 10.7507/1002-1892.202503013. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2025. PMID: 40830133 Free PMC article. Chinese.
-
Another Notch in the Belt of Rheumatoid Arthritis.Arthritis Rheumatol. 2024 Oct;76(10):1475-1487. doi: 10.1002/art.42937. Epub 2024 Aug 9. Arthritis Rheumatol. 2024. PMID: 38961731 Review.
-
Indigenous Nigeria medicinal herbal remedies: A potential source for therapeutic against rheumatoid arthritis.Exp Biol Med (Maywood). 2022 Jul;247(13):1148-1178. doi: 10.1177/15353702221102901. Epub 2022 Jun 16. Exp Biol Med (Maywood). 2022. PMID: 35708153 Free PMC article. Review.
Cited by
-
Anti-Inflammatory Effects of Hydroethanolic Extract from Ehretia asperula on Lipopolysaccharide-Stimulated RAW264.7 Macrophages.J Microbiol Biotechnol. 2024 Jun 28;34(6):1340-1347. doi: 10.4014/jmb.2403.03006. Epub 2024 Apr 15. J Microbiol Biotechnol. 2024. PMID: 38783718 Free PMC article.
-
Triptonoterpene, a Natural Product from Celastrus orbiculatus Thunb, Has Biological Activity against the Metastasis of Gastric Cancer Cells.Molecules. 2022 Nov 18;27(22):8005. doi: 10.3390/molecules27228005. Molecules. 2022. PMID: 36432106 Free PMC article.
-
Targeting matrix metalloproteases: A promising strategy for herbal medicines to treat rheumatoid arthritis.Front Immunol. 2022 Nov 9;13:1046810. doi: 10.3389/fimmu.2022.1046810. eCollection 2022. Front Immunol. 2022. PMID: 36439173 Free PMC article. Review.
-
Celastrus orbiculatus extracts induce cell cycle arrest and apoptosis in human esophageal squamous carcinoma ECA-109 cells in vitro via the PI3K/AKT/mTOR signaling pathway.Oncol Lett. 2018 Feb;15(2):1591-1599. doi: 10.3892/ol.2017.7459. Epub 2017 Nov 21. Oncol Lett. 2018. PMID: 29434854 Free PMC article.
-
Phytochemicals as potential antidotes for targeting NF-κB in rheumatoid arthritis.3 Biotech. 2017 Aug;7(4):253. doi: 10.1007/s13205-017-0888-1. Epub 2017 Jul 18. 3 Biotech. 2017. PMID: 28721679 Free PMC article. Review.
References
-
- Lefevre S, Knedla A, Tennie C, Kampmann A, Wunrau C, Dinser R, Korb A, Schnaker EM, Tarner IH, Robbins PD, Evans CH, Sturz H, Steinmeyer J, Gay S, Scholmerich J, Pap T, Muller-Ladner U, Neumann E. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009;15:1414–1420. doi: 10.1038/nm.2050. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

