IN.PACT Amphirion paclitaxel eluting balloon versus standard percutaneous transluminal angioplasty for infrapopliteal revascularization of critical limb ischemia: rationale and protocol for an ongoing randomized controlled trial
- PMID: 24552184
- PMCID: PMC3936931
- DOI: 10.1186/1745-6215-15-63
IN.PACT Amphirion paclitaxel eluting balloon versus standard percutaneous transluminal angioplasty for infrapopliteal revascularization of critical limb ischemia: rationale and protocol for an ongoing randomized controlled trial
Abstract
Background: The effectiveness and durability of endovascular revascularization therapies for chronic critical limb ischemia (CLI) are challenged by the extensive burden of infrapopliteal arterial disease and lesion-related characteristics (e.g., severe calcification, chronic total occlusions), which frequently result in poor clinical outcomes. While infrapopliteal vessel patency directly affects pain relief and wound healing, sustained patency and extravascular care both contribute to the ultimate "patient-centric" outcomes of functional limb preservation, mobility and quality of life (QoL).
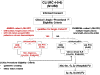
Methods/design: IN.PACT DEEP is a 2:1 randomized controlled trial designed to assess the efficacy and safety of infrapopliteal arterial revascularization between the IN.PACT Amphirion™ paclitaxel drug-eluting balloon (IA-DEB) and standard balloon angioplasty (PTA) in patients with Rutherford Class 4-5-6 CLI.
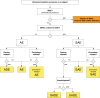
Discussion: This multicenter trial has enrolled 358 patients at 13 European centers with independent angiographic core lab adjudication of the primary efficacy endpoint of target lesion late luminal loss (LLL) and clinically driven target lesion revascularization (TLR) in major amputation-free surviving patients through 12-months. An independent wound core lab will evaluate all ischemic wounds to assess the extent of healing and time to healing at 1, 6, and 12 months. A QoL questionnaire including a pain scale will assess changes from baseline scores through 12 months. A Clinical Events Committee and Data Safety Monitoring Board will adjudicate the composite primary safety endpoints of all-cause death, major amputation, and clinically driven TLR at 6 months and other trial endpoints and supervise patient safety throughout the study. All patients will be followed for 5 years. A literature review is presented of the current status of endovascular treatment of CLI with drug-eluting balloon and standard PTA. The rationale and design of the IN.PACT DEEP Trial are discussed. IN.PACT DEEP is a milestone, prospective, randomized, robust, independent core lab-adjudicated CLI trial that will evaluate the role of a new infrapopliteal revascularization technology, the IA-DEB, compared to PTA. It will assess the overall impact on infrapopliteal artery patency, limb salvage, wound healing, pain control, QoL, and patient mobility. The 1-year results of the adjudicated co-primary and secondary endpoints will be available in 2014.
Trial registration: NCT00941733.
Figures
References
-
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H. BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicenter, randomised controlled trial. Lancet. 2005;366(9501):1925–1934. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous