Diastereoselective synthesis of γ- and δ-lactams from imines and sulfone-substituted anhydrides
- PMID: 24552208
- PMCID: PMC3977582
- DOI: 10.1021/jo500050n
Diastereoselective synthesis of γ- and δ-lactams from imines and sulfone-substituted anhydrides
Abstract
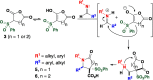
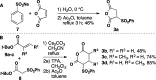
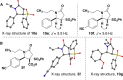
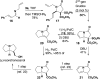
Sulfone-substituted γ- and δ-lactams have been prepared in a single step with high diastereoselectivity. Sulfonylglutaric anhydrides produce intermediates that readily decarboxylate to provide δ-lactams with high diastereoselectivity. Substituents at the 3- or 4-position of the glutaric anhydride induce high levels of stereocontrol. Sulfonylsuccinic anhydrides produce intermediate carboxylic acids that can be trapped as methyl esters or allowed to decarboxylate under mild conditions. This method has been applied to a short synthesis of the pyrrolizidine alkaloid (±)-isoretronecanol.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

