Long-term oncological outcomes of a phase II trial of neoadjuvant chemohormonal therapy followed by radical prostatectomy for patients with clinically localised, high-risk prostate cancer
- PMID: 24552276
- PMCID: PMC4219923
- DOI: 10.1111/bju.12676
Long-term oncological outcomes of a phase II trial of neoadjuvant chemohormonal therapy followed by radical prostatectomy for patients with clinically localised, high-risk prostate cancer
Abstract
Objective: To determine long-term oncological outcomes of radical prostatectomy (RP) after neoadjuvant chemohormonal therapy (CHT) for clinically localised, high-risk prostate cancer.
Patients and methods: In this phase II multicentre trial of patients with high-risk prostate cancer (PSA level >20 ng/mL, Gleason ≥8, or clinical stage ≥T3), androgen-deprivation therapy (goserelin acetate depot) and paclitaxel, carboplatin and estramustine were administered before RP. We report the long-term oncological outcomes of these patients and compared them to a contemporary cohort who met oncological inclusion criteria but received RP only.
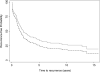
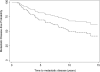
Results: In all, 34 patients were enrolled and followed for a median of 13.1 years. Within 10 years most patients had biochemical recurrence (BCR-free probability 22%; 95% confidence interval [CI] 10-37%). However, the probability of disease-specific survival at 10 years was 84% (95% CI 66-93%) and overall survival was 78% (95% CI 60-89%). The CHT group had higher-risk features than the comparison group (123 patients), with an almost doubled risk of calculated preoperative 5-year BCR (69% vs 36%, P < 0.01). After adjusting for these imbalances the CHT group had trends toward improvement in BCR (hazard ratio [HR] 0.76, 95% CI 0.43-1.34; P = 0.3) and metastasis-free survival (HR 0.55, 95% CI 0.24-1.29; P = 0.2) although these were not statistically significant.
Conclusions: Neoadjuvant CHT followed by RP was associated with lower rates of BCR and metastasis compared with the RP-only group; however, these results were not statistically significant. Because this treatment strategy has known harms and unproven benefit, this strategy should only be instituted in the setting of a clinical trial.
Keywords: chemotherapy; hormone therapy; neoadjuvant; prostate neoplasms; radical prostatectomy; survival.
© 2014 The Authors BJU International © 2014 BJU International Published by John Wiley & Sons Ltd.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. 2012 - PubMed
-
- Yossepowitch O, Eggener SE, Bianco FJ, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. The Journal of urology. 2007;178(2):493–9. - PubMed
-
- Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10(9):1081–7. - PubMed
-
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

