Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiments
- PMID: 24552401
- PMCID: PMC3979472
- DOI: 10.1021/jp412051v
Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiments
Abstract
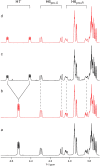
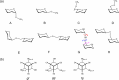
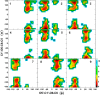
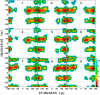
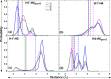
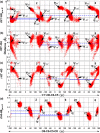
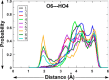
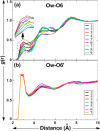
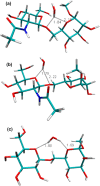
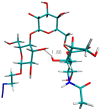
Conformational sampling for a set of 10 α- or β-(1→6)-linked oligosaccharides has been studied using explicit solvent Hamiltonian replica exchange (HREX) simulations and NMR spectroscopy techniques. Validation of the force field and simulation methodology is done by comparing calculated transglycosidic J coupling constants and proton-proton distances with the corresponding NMR data. Initial calculations showed poor agreement, for example, with >3 Hz deviation of the calculated (3)J(H5,H6R) values from the experimental data, prompting optimization of the ω torsion angle parameters associated with (1→6)-linkages. The resulting force field is in overall good agreement (i.e., within ∼0.5 Hz deviation) from experimental (3)J(H5,H6R) values, although some small limitations are evident. Detailed hydrogen bonding analysis indicates that most of the compounds lack direct intramolecular H-bonds between the two monosaccharides; however, minor sampling of the O6···HO2' hydrogen bond is present in three compounds. The results verify the role of the gauche effect between O5 and O6 atoms in gluco- and manno-configured pyranosides causing the ω torsion angle to sample an equilibrium between the gt and gg rotamers. Conversely, galacto-configured pyranosides sample a population distribution in equilibrium between gt and tg rotamers, while the gg rotamer populations are minor. Water radial distribution functions suggest decreased accessibility to the O6 atom in the (1→6)-linkage as compared to the O6' atom in the nonreducing sugar. The role of bridging water molecules between two sugar moieties on the distributions of ω torsion angles in oligosaccharides is also explored.
Figures



Similar articles
-
Conformational properties of methyl β-maltoside and methyl α- and β-cellobioside disaccharides.J Phys Chem B. 2011 Jan 27;115(3):597-608. doi: 10.1021/jp109475p. Epub 2010 Dec 15. J Phys Chem B. 2011. PMID: 21158455 Free PMC article.
-
Molecular conformations in the pentasaccharide LNF-1 derived from NMR spectroscopy and molecular dynamics simulations.J Phys Chem B. 2011 Jun 2;115(21):7109-21. doi: 10.1021/jp2017105. Epub 2011 May 5. J Phys Chem B. 2011. PMID: 21545157
-
Conformational flexibility and dynamics of two (1-->6)-linked disaccharides related to an oligosaccharide epitope expressed on malignant tumour cells.Chemistry. 2009 Sep 7;15(35):8886-94. doi: 10.1002/chem.200900507. Chemistry. 2009. PMID: 19637158
-
Conformational Preferences at the Glycosidic Linkage of Saccharides in Solution as Deduced from NMR Experiments and MD Simulations: Comparison to Crystal Structures.Chemistry. 2024 Mar 12;30(15):e202304047. doi: 10.1002/chem.202304047. Epub 2024 Jan 24. Chemistry. 2024. PMID: 38180821
-
A perspective on the primary and three-dimensional structures of carbohydrates.Carbohydr Res. 2013 Aug 30;378:123-32. doi: 10.1016/j.carres.2013.02.005. Epub 2013 Feb 24. Carbohydr Res. 2013. PMID: 23522728 Review.
Cited by
-
Unveiling Molecular Recognition of Sialoglycans by Human Siglec-10.iScience. 2020 Jun 26;23(6):101231. doi: 10.1016/j.isci.2020.101231. Epub 2020 Jun 2. iScience. 2020. PMID: 32629603 Free PMC article.
-
Escherichia coli O176 LPS structure and dynamics: A NMR spectroscopy and MD simulation study.Curr Res Struct Biol. 2020 Apr 22;2:79-88. doi: 10.1016/j.crstbi.2020.04.004. eCollection 2020. Curr Res Struct Biol. 2020. PMID: 34235471 Free PMC article.
-
Delineating the conformational flexibility of trisaccharides from NMR spectroscopy experiments and computer simulations.Phys Chem Chem Phys. 2016 Jul 28;18(28):18776-94. doi: 10.1039/c6cp02970a. Epub 2016 Jun 27. Phys Chem Chem Phys. 2016. PMID: 27346493 Free PMC article.
-
Glycosidic α-linked mannopyranose disaccharides: an NMR spectroscopy and molecular dynamics simulation study employing additive and Drude polarizable force fields.Phys Chem Chem Phys. 2023 Jan 27;25(4):3042-3060. doi: 10.1039/d2cp05203b. Phys Chem Chem Phys. 2023. PMID: 36607620 Free PMC article.
-
CHARMM Drude Polarizable Force Field for Glycosidic Linkages Involving Pyranoses and Furanoses.J Chem Theory Comput. 2018 Jun 12;14(6):3132-3143. doi: 10.1021/acs.jctc.8b00175. Epub 2018 May 4. J Chem Theory Comput. 2018. PMID: 29694037 Free PMC article.
References
-
- Dwek R. A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683–720. - PubMed
-
- Dwek R. A.; Butters T. D. Introduction: Glycobiology – Understanding the Language and Meaning of Carbohydrates. Chem. Rev. 2002, 102, 283–284.
-
- El Kadib A.; Bousmina M. Chitosan Bio-Based Organic–Inorganic Hybrid Aerogel Microspheres. Chem.—Eur. J. 2012, 18, 8264–8277. - PubMed
-
- Koutsopoulos S. Molecular Fabrications of Smart Nanobiomaterials and Applications in Personalized Medicine. Adv. Drug Delivery Rev. 2012, 64, 1459–1476. - PubMed
-
- Slaney A. M.; Wright V. A.; Meloncelli P. J.; Harris K. D.; West L. J.; Lowary T. L.; Buriak J. M. Biocompatible Carbohydrate-Functionalized Stainless Steel Surfaces: A New Method for Passivating Biomedical Implants. ACS Appl. Mater. Interfaces 2011, 3, 1601–1612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

