Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiments
- PMID: 24552401
- PMCID: PMC3979472
- DOI: 10.1021/jp412051v
Conformational properties of α- or β-(1→6)-linked oligosaccharides: Hamiltonian replica exchange MD simulations and NMR experiments
Abstract
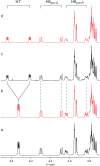
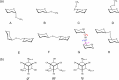
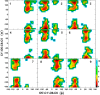
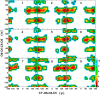
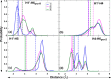
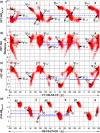
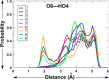
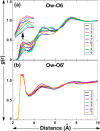
Conformational sampling for a set of 10 α- or β-(1→6)-linked oligosaccharides has been studied using explicit solvent Hamiltonian replica exchange (HREX) simulations and NMR spectroscopy techniques. Validation of the force field and simulation methodology is done by comparing calculated transglycosidic J coupling constants and proton-proton distances with the corresponding NMR data. Initial calculations showed poor agreement, for example, with >3 Hz deviation of the calculated (3)J(H5,H6R) values from the experimental data, prompting optimization of the ω torsion angle parameters associated with (1→6)-linkages. The resulting force field is in overall good agreement (i.e., within ∼0.5 Hz deviation) from experimental (3)J(H5,H6R) values, although some small limitations are evident. Detailed hydrogen bonding analysis indicates that most of the compounds lack direct intramolecular H-bonds between the two monosaccharides; however, minor sampling of the O6···HO2' hydrogen bond is present in three compounds. The results verify the role of the gauche effect between O5 and O6 atoms in gluco- and manno-configured pyranosides causing the ω torsion angle to sample an equilibrium between the gt and gg rotamers. Conversely, galacto-configured pyranosides sample a population distribution in equilibrium between gt and tg rotamers, while the gg rotamer populations are minor. Water radial distribution functions suggest decreased accessibility to the O6 atom in the (1→6)-linkage as compared to the O6' atom in the nonreducing sugar. The role of bridging water molecules between two sugar moieties on the distributions of ω torsion angles in oligosaccharides is also explored.
Figures



References
-
- Dwek R. A. Glycobiology: Toward Understanding the Function of Sugars. Chem. Rev. 1996, 96, 683–720. - PubMed
-
- Dwek R. A.; Butters T. D. Introduction: Glycobiology – Understanding the Language and Meaning of Carbohydrates. Chem. Rev. 2002, 102, 283–284.
-
- El Kadib A.; Bousmina M. Chitosan Bio-Based Organic–Inorganic Hybrid Aerogel Microspheres. Chem.—Eur. J. 2012, 18, 8264–8277. - PubMed
-
- Koutsopoulos S. Molecular Fabrications of Smart Nanobiomaterials and Applications in Personalized Medicine. Adv. Drug Delivery Rev. 2012, 64, 1459–1476. - PubMed
-
- Slaney A. M.; Wright V. A.; Meloncelli P. J.; Harris K. D.; West L. J.; Lowary T. L.; Buriak J. M. Biocompatible Carbohydrate-Functionalized Stainless Steel Surfaces: A New Method for Passivating Biomedical Implants. ACS Appl. Mater. Interfaces 2011, 3, 1601–1612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

