Chitosan/siRNA nanoparticle targeting demonstrates a requirement for single-minded during larval and pupal olfactory system development of the vector mosquito Aedes aegypti
- PMID: 24552425
- PMCID: PMC3936921
- DOI: 10.1186/1471-213X-14-9
Chitosan/siRNA nanoparticle targeting demonstrates a requirement for single-minded during larval and pupal olfactory system development of the vector mosquito Aedes aegypti
Abstract
Background: Essentially nothing is known about the genetic regulation of olfactory system development in vector mosquitoes, which use olfactory cues to detect blood meal hosts. Studies in Drosophila melanogaster have identified a regulatory matrix of transcription factors that controls pupal/adult odorant receptor (OR) gene expression in olfactory receptor neurons (ORNs). However, it is unclear if transcription factors that function in the D. melanogaster regulatory matrix are required for OR expression in mosquitoes. Furthermore, the regulation of OR expression during development of the larval olfactory system, which is far less complex than that of pupae/adults, is not well understood in any insect, including D. melanogaster. Here, we examine the regulation of OR expression in the developing larval olfactory system of Aedes aegypti, the dengue vector mosquito.
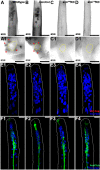
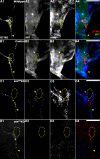
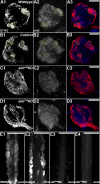
Results: A. aegypti bears orthologs of eight transcription factors that regulate OR expression in D. melanogaster pupae/adults. These transcription factors are expressed in A. aegypti larval antennal sensory neurons, and consensus binding sites for these transcription factors reside in the 5' flanking regions of A. aegypti OR genes. Consensus binding sites for Single-minded (Sim) are located adjacent to over half the A. aegypti OR genes, suggesting that this transcription factor functions as a major regulator of mosquito OR expression. To functionally test this hypothesis, chitosan/siRNA nanoparticles were used to target sim during larval olfactory development. These experiments demonstrated that Sim positively regulates expression of a large subset of OR genes, including orco, the obligate co-receptor in the assembly and function of heteromeric OR/Orco complexes. Decreased innervation of the antennal lobe was also noted in sim knockdown larvae. These OR expression and antennal lobe defects correlated with a larval odorant tracking behavioral defect. OR expression and antennal lobe defects were also observed in sim knockdown pupae.
Conclusions: The results of this investigation indicate that Sim has multiple functions during larval and pupal olfactory system development in A. aegypti.
Figures





Similar articles
-
Disruption of Aedes aegypti olfactory system development through chitosan/siRNA nanoparticle targeting of semaphorin-1a.PLoS Negl Trop Dis. 2013 May 16;7(5):e2215. doi: 10.1371/journal.pntd.0002215. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23696908 Free PMC article.
-
Knockout of juvenile hormone receptor, Methoprene-tolerant, induces black larval phenotype in the yellow fever mosquito, Aedes aegypti.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21501-21507. doi: 10.1073/pnas.1905729116. Epub 2019 Sep 30. Proc Natl Acad Sci U S A. 2019. PMID: 31570611 Free PMC article.
-
Identification of Aedes aegypti cis-regulatory elements that promote gene expression in olfactory receptor neurons of distantly related dipteran insects.Parasit Vectors. 2018 Jul 11;11(1):406. doi: 10.1186/s13071-018-2982-6. Parasit Vectors. 2018. PMID: 29996889 Free PMC article.
-
Evolution, developmental expression and function of odorant receptors in insects.J Exp Biol. 2020 Feb 7;223(Pt Suppl 1):jeb208215. doi: 10.1242/jeb.208215. J Exp Biol. 2020. PMID: 32034042 Free PMC article. Review.
-
Prospects on non-canonical olfaction in the mosquito and other organisms: why co-express?Curr Opin Insect Sci. 2025 Feb;67:101291. doi: 10.1016/j.cois.2024.101291. Epub 2024 Oct 28. Curr Opin Insect Sci. 2025. PMID: 39471910 Review.
Cited by
-
Challenges of Robust RNAi-Mediated Gene Silencing in Aedes Mosquitoes.Int J Mol Sci. 2024 May 10;25(10):5218. doi: 10.3390/ijms25105218. Int J Mol Sci. 2024. PMID: 38791257 Free PMC article.
-
Preparation and Use of a Yeast shRNA Delivery System for Gene Silencing in Mosquito Larvae.Methods Mol Biol. 2019;1858:213-231. doi: 10.1007/978-1-4939-8775-7_15. Methods Mol Biol. 2019. PMID: 30414120 Free PMC article.
-
RNAi turns 25:contributions and challenges in insect science.Front Insect Sci. 2023 Oct 4;3:1209478. doi: 10.3389/finsc.2023.1209478. eCollection 2023. Front Insect Sci. 2023. PMID: 38469536 Free PMC article. Review.
-
Simultaneous silencing of juvenile hormone metabolism genes through RNAi interrupts metamorphosis in the cotton boll weevil.Front Mol Biosci. 2023 Mar 6;10:1073721. doi: 10.3389/fmolb.2023.1073721. eCollection 2023. Front Mol Biosci. 2023. PMID: 36950526 Free PMC article.
-
RNA Interference in Insects: Protecting Beneficials and Controlling Pests.Front Physiol. 2019 Jan 11;9:1912. doi: 10.3389/fphys.2018.01912. eCollection 2018. Front Physiol. 2019. PMID: 30687124 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

