Role of hepatic and intestinal p450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice
- PMID: 24552495
- PMCID: PMC4002058
- DOI: 10.1021/tx4004769
Role of hepatic and intestinal p450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice
Abstract
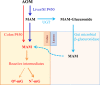
P450-mediated bioactivation of azoxymethane (AOM), a colon carcinogen, leads to the formation of DNA adducts, of which O(6)-methylguanine (O(6)-mG) is the most mutagenic and contributes to colon tumorigenesis. To determine whether P450 enzymes of the liver and intestine both contribute to AOM bioactivation in vivo, we compared tissue levels of AOM-induced DNA adducts, microsomal AOM metabolic activities, and incidences of colonic aberrant crypt foci (ACF) among wild-type (WT), liver-specific P450 reductase (Cpr)-null (LCN), and intestinal epithelium-specific Cpr-null (IECN) mice. At 6 h following AOM treatment (at 14 mg/kg, s.c.), O(6)-mG and N(7)-mG levels were highest in the liver, followed by the colon, and then small intestine in WT mice. As expected, hepatic adduct levels were significantly lower (by >60%) in LCN mice but unchanged in IECN mice, whereas small-intestinal adduct levels were unchanged or increased in LCN mice but lower (by >50%) in IECN mice compared to that in WT mice. However, colonic adduct levels were unchanged in IECN mice compared to that in WT mice and increased in LCN mice (by 1.5-2.9-fold). The tissue-specific impact of the CPR loss in IECN and LCN mice on microsomal AOM metabolic activity was confirmed by rates of formation of formaldehyde and N(7)-mG in vitro. Furthermore, the incidence of ACF, a lesion preceding colon cancer, was similar in the three mouse strains. Thus, AOM-induced colonic DNA damage and ACF formation is not solely dependent on either hepatic or intestinal microsomal P450 enzymes. P450 enzymes in both the liver and intestine likely contribute to AOM-induced colon carcinogenesis.
Figures


Similar articles
-
Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol.Cancer Res. 2001 Dec 1;61(23):8435-40. Cancer Res. 2001. PMID: 11731424
-
Effect of Cytochrome P450 Reductase Deficiency on 2-Amino-9H-pyrido[2,3-b]indole Metabolism and DNA Adduct Formation in Liver and Extrahepatic Tissues of Mice.Chem Res Toxicol. 2015 Dec 21;28(12):2400-10. doi: 10.1021/acs.chemrestox.5b00405. Epub 2015 Dec 3. Chem Res Toxicol. 2015. PMID: 26583703 Free PMC article.
-
Comparative DNA adduct formation and induction of colonic aberrant crypt foci in mice exposed to 2-amino-9H-pyrido[2,3-b]indole, 2-amino-3,4-dimethylimidazo[4,5-f]quinoline, and azoxymethane.Environ Mol Mutagen. 2016 Mar;57(2):125-36. doi: 10.1002/em.21993. Epub 2016 Jan 6. Environ Mol Mutagen. 2016. PMID: 26734915 Free PMC article.
-
Regulation of intestinal cytochrome P450 expression by hepatic cytochrome P450: possible involvement of fibroblast growth factor 15 and impact on systemic drug exposure.Mol Pharmacol. 2014 Jan;85(1):139-47. doi: 10.1124/mol.113.088914. Epub 2013 Nov 1. Mol Pharmacol. 2014. PMID: 24184963 Free PMC article.
-
An update on the role of intestinal cytochrome P450 enzymes in drug disposition.Acta Pharm Sin B. 2016 Sep;6(5):374-383. doi: 10.1016/j.apsb.2016.07.012. Epub 2016 Aug 4. Acta Pharm Sin B. 2016. PMID: 27709006 Free PMC article. Review.
Cited by
-
Thai Fermented Soybean (Thua-Nao) Prevents Early Stages of Colorectal Carcinogenesis Induced by Diethylnitrosamine and 1,2-Dimethylhydrazine Through Modulations of Cell Proliferation and Gut Microbiota in Rats.Nutrients. 2024 Oct 16;16(20):3506. doi: 10.3390/nu16203506. Nutrients. 2024. PMID: 39458500 Free PMC article.
-
Mapping insoluble indole metabolites in the gastrointestinal environment of a murine colorectal cancer model using desorption/ionisation on porous silicon imaging.Sci Rep. 2019 Aug 26;9(1):12342. doi: 10.1038/s41598-019-48533-2. Sci Rep. 2019. PMID: 31451756 Free PMC article.
-
Tucum-do-cerrado (Bactris setosa Mart.) modulates oxidative stress, inflammation, and apoptosis-related proteins in rats treated with azoxymethane.PLoS One. 2018 Nov 14;13(11):e0206670. doi: 10.1371/journal.pone.0206670. eCollection 2018. PLoS One. 2018. PMID: 30427888 Free PMC article.
-
Orientin, a flavanoid, mitigates 1, 2 dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet.Toxicol Rep. 2018 Sep 27;5:977-987. doi: 10.1016/j.toxrep.2018.09.004. eCollection 2018. Toxicol Rep. 2018. PMID: 30319939 Free PMC article.
-
Experimental Murine Models for Colorectal Cancer Research.Cancers (Basel). 2023 Apr 30;15(9):2570. doi: 10.3390/cancers15092570. Cancers (Basel). 2023. PMID: 37174036 Free PMC article. Review.
References
-
- Femia A. P.; Caderni G. (2008) Rodent models of colon carcinogenesis for the study of chemopreventive activity of natural products. Planta Med. 74, 1602–1607. - PubMed
-
- Magnuson B.; South E.; Exon J.; Dashwood R.; Xu M.; Hendrix K.; Hubele S. (2000) Increased susceptibility of adult rats to azoxymethane-induced aberrant crypt foci. Cancer Lett. 161, 185–193. - PubMed
-
- Perše M.; Cerar A. (2005) The dimethylhydrazine induced colorectal tumours in rat-experimental colorectal carcinogenesis. Radiol. Oncol. 39, 61–70.
-
- Corpet D. E.; Pierre F. (2005) How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur. J. Cancer 41, 1911–1922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

