SmgGDS-558 regulates the cell cycle in pancreatic, non-small cell lung, and breast cancers
- PMID: 24552806
- PMCID: PMC3984317
- DOI: 10.4161/cc.27804
SmgGDS-558 regulates the cell cycle in pancreatic, non-small cell lung, and breast cancers
Abstract
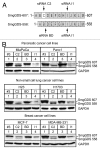
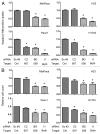
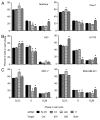
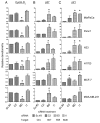
Oncogenic mutation or misregulation of small GTPases in the Ras and Rho families can promote unregulated cell cycle progression in cancer. Post-translational modification by prenylation of these GTPases allows them to signal at the cell membrane. Splice variants of SmgGDS, named SmgGDS-607 and SmgGDS-558, promote the prenylation and membrane trafficking of multiple Ras and Rho family members, which makes SmgGDS a potentially important regulator of the cell cycle. Surprisingly little is known about how SmgGDS-607 and SmgGDS-558 affect cell cycle-regulatory proteins in cancer, even though SmgGDS is overexpressed in multiple types of cancer. To examine the roles of SmgGDS splice variants in the cell cycle, we compared the effects of the RNAi-mediated depletion of SmgGDS-558 vs. SmgGDS-607 on cell cycle progression and the expression of cyclin D1, p27, and p21 in pancreatic, lung, and breast cancer cell lines. We show for the first time that SmgGDS promotes proliferation of pancreatic cancer cells, and we demonstrate that SmgGDS-558 plays a greater role than SmgGDS-607 in cell cycle progression as well as promoting cyclin D1 and suppressing p27 expression in multiple types of cancer. Silencing both splice variants of SmgGDS in the cancer cell lines produces an alternative signaling profile compared with silencing SmgGDS-558 alone. We also show that loss of both SmgGDS-607 and SmgGDS-558 simultaneously decreases tumorigenesis of NCI-H1703 non-small cell lung carcinoma (NSCLC) xenografts in mice. These findings indicate that SmgGDS promotes cell cycle progression in multiple types of cancer, making SmgGDS a valuable target for cancer therapeutics.
Keywords: GTPase; RNAi; Rap1GDS1; SmgGDS; breast cancer; cell cycle; lung cancer; mouse tumorigenesis; pancreatic cancer; proliferation.
Figures
References
-
- Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Nakamura H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001;61:4885–91. - PubMed
-
- Taniuchi K, Nakagawa H, Hosokawa M, Nakamura T, Eguchi H, Ohigashi H, Ishikawa O, Katagiri T, Nakamura Y. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005;65:3092–9. - PubMed
-
- Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, et al. Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer. 2009;63:375–82. doi: 10.1016/j.lungcan.2008.12.012. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
