Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease
- PMID: 24552807
- PMCID: PMC3984313
- DOI: 10.4161/cc.28255
Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease
Abstract
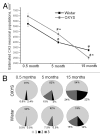
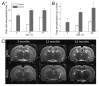
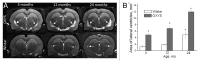
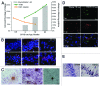
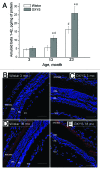
Senescence-accelerated OXYS rats are an experimental model of accelerated aging that was established from Wistar stock via selection for susceptibility to cataractogenic effects of a galactose-rich diet and via subsequent inbreeding of highly susceptible rats. Currently, we have the 102nd generation of OXYS rats with spontaneously developing cataract and accelerated senescence syndrome, which means early development of a phenotype similar to human geriatric disorders, including accelerated brain aging. In recent years, our group found strong evidence that OXYS rats are a promising model for studies of the mechanisms of brain aging and neurodegenerative processes similar to those seen in Alzheimer disease (AD). The manifestation of behavioral alterations and learning and memory deficits develop since the fourth week of age, i.e., simultaneously with first signs of neurodegeneration detectable on magnetic resonance imaging and under a light microscope. In addition, impaired long-term potentiation has been demonstrated in OXYS rats by the age of 3 months. With age, neurodegenerative changes in the brain of OXYS rats become amplified. We have shown that this deterioration happens against the background of overproduction of amyloid precursor protein (AβPP), accumulation of β-amyloid (Aβ), and hyperphosphorylation of the tau protein in the hippocampus and cortex. The development of AMD-like retinopathy in OXYS rats is also accompanied by increased accumulation of Aβ in the retina. These published data suggest that the OXYS strain may serve as a spontaneous rat model of AD-like pathology and could help to decipher the pathogenesis of AD.
Keywords: Alzheimer disease; brain aging; senescence-accelerated OXYS rats.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
