Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link
- PMID: 24552811
- PMCID: PMC3984312
- DOI: 10.4161/cc.28189
Mitochondrial alteration in type 2 diabetes and obesity: an epigenetic link
Abstract
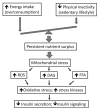
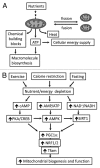
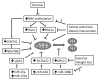
The growing epidemic of type 2 diabetes mellitus (T2DM) and obesity is largely attributed to the current lifestyle of over-consumption and physical inactivity. As the primary platform controlling metabolic and energy homeostasis, mitochondria show aberrant changes in T2DM and obese subjects. While the underlying mechanism is under extensive investigation, epigenetic regulation is now emerging to play an important role in mitochondrial biogenesis, function, and dynamics. In line with lifestyle modifications preventing mitochondrial alterations and metabolic disorders, exercise has been shown to change DNA methylation of the promoter of PGC1α to favor gene expression responsible for mitochondrial biogenesis and function. In this article we discuss the epigenetic mechanism of mitochondrial alteration in T2DM and obesity, and the effects of lifestyle on epigenetic regulation. Future studies designed to further explore and integrate the epigenetic mechanisms with lifestyle modification may lead to interdisciplinary interventions and novel preventive options for mitochondrial alteration and metabolic disorders.
Keywords: epigenetic; lifestyle; mitochondrial alteration; obesity; type 2 diabetes.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
