Distinctive effects of the cellular inhibitor of apoptosis protein c-IAP2 through stabilization by XIAP in glioblastoma multiforme cells
- PMID: 24552816
- PMCID: PMC3984322
- DOI: 10.4161/cc.27880
Distinctive effects of the cellular inhibitor of apoptosis protein c-IAP2 through stabilization by XIAP in glioblastoma multiforme cells
Abstract
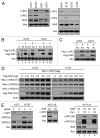
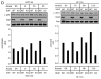
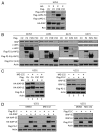
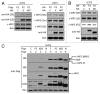
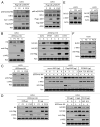
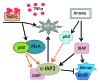
Inhibitor of apoptosis proteins (IAPs) are extensively involved in NFκB signaling pathways. Regulation of c-IAP2 turnover by other proteins was investigated in glioblastoma multiforme (GBM) cells in the present study. When overexpressed, X-linked IAP (XIAP) enhanced expression of ectopic c-IAP2, but not c-IAP1, and endogenous c-IAP2 levels were reduced once XIAP expression was silenced. TNFα stimulation substantially increased c-IAP2 expression, and this upregulation was impaired by suppression of XIAP. Similarly, when XIAP was limiting due to severe hypoxic conditions, c-IAP2 levels were downregulated. These data together indicate that XIAP is an important regulator responsible for stabilization of c-IAP2 levels under different conditions. Protein interactions occur through binding of BIR2 and BIR3 domains of c-IAP2 with the RING finger of XIAP. XIAP inhibition of c-IAP2 auto-degradation was dependent on this physical interaction, and it was independent of XIAP E3 ligase activity. Global c-IAP2 ubiquitination was not affected by XIAP, although c-IAP2 levels were significantly increased. A CARD-RING-containing fragment of c-IAP2 was found to target XIAP for proteasome-independent degradation, but it was unable to sensitize GBM cells to chemo-reagents. The XIAP-stabilized c-IAP2 was found to enhance IκB-α phosphorylation on serines 32 and 36, and to antagonize XIAP-induced increase in mature Smac and Bcl10. Taken together, our data identify a distinctive role of c-IAP2 as stabilizer of XIAP, which is likely involved in regulation of NFκB activation and apoptosis in GBM cells.
Keywords: Bcl10; IκB-α; TNFα; XIAP; anoxia; auto-degradation; c-IAP2; glioblastoma multiforme cell lines; mature Smac.
Figures




Similar articles
-
Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1).Biochem J. 2009 Jan 1;417(1):149-60. doi: 10.1042/BJ20081885. Biochem J. 2009. PMID: 18939944
-
c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation.J Biol Chem. 2008 Sep 5;283(36):24295-9. doi: 10.1074/jbc.C800128200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621737 Free PMC article.
-
Identification of non-canonical NF-κB signaling as a critical mediator of Smac mimetic-stimulated migration and invasion of glioblastoma cells.Cell Death Dis. 2013 Mar 28;4(3):e564. doi: 10.1038/cddis.2013.70. Cell Death Dis. 2013. PMID: 23538445 Free PMC article.
-
IAP family of cell death and signaling regulators.Methods Enzymol. 2014;545:35-65. doi: 10.1016/B978-0-12-801430-1.00002-0. Methods Enzymol. 2014. PMID: 25065885 Review.
-
(Un)expected roles of c-IAPs in apoptotic and NFkappaB signaling pathways.Cell Cycle. 2008 Jun 1;7(11):1511-21. doi: 10.4161/cc.7.11.5959. Epub 2008 Mar 16. Cell Cycle. 2008. PMID: 18469528 Review.
Cited by
-
Immunohistochemical Characterization of Procaspase-3 Overexpression as a Druggable Target With PAC-1, a Procaspase-3 Activator, in Canine and Human Brain Cancers.Front Oncol. 2019 Feb 25;9:96. doi: 10.3389/fonc.2019.00096. eCollection 2019. Front Oncol. 2019. PMID: 30859090 Free PMC article.
-
X-linked inhibitor of apoptosis protein (XIAP) lacking RING domain localizes to the nuclear and promotes cancer cell anchorage-independent growth by targeting the E2F1/Cyclin E axis.Oncotarget. 2014 Aug 30;5(16):7126-37. doi: 10.18632/oncotarget.2227. Oncotarget. 2014. PMID: 25216527 Free PMC article.
-
A small-molecule ARTS mimetic promotes apoptosis through degradation of both XIAP and Bcl-2.Cell Death Dis. 2020 Jun 25;11(6):483. doi: 10.1038/s41419-020-2670-2. Cell Death Dis. 2020. PMID: 32587235 Free PMC article.
-
BIRC3 is a novel driver of therapeutic resistance in Glioblastoma.Sci Rep. 2016 Feb 18;6:21710. doi: 10.1038/srep21710. Sci Rep. 2016. PMID: 26888114 Free PMC article.
-
Combination of quercetin and cisplatin enhances apoptosis in OSCC cells by downregulating xIAP through the NF-κB pathway.J Cancer. 2019 Jul 25;10(19):4509-4521. doi: 10.7150/jca.31045. eCollection 2019. J Cancer. 2019. PMID: 31528215 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
