Cardiopulmonary coupling in chronic obstructive pulmonary disease: the role of imaging
- PMID: 24552883
- PMCID: PMC4274316
- DOI: 10.1097/RTI.0000000000000076
Cardiopulmonary coupling in chronic obstructive pulmonary disease: the role of imaging
Abstract
Chronic obstructive pulmonary disorder (COPD) is a systemic disease that affects the cardiovascular system through multiple pathways. Pulmonary hypertension, ventricular dysfunction, and atherosclerosis are associated with smoking and COPD, causing significant morbidity and poor prognosis. Coupling between the pulmonary and cardiovascular system involves mechanical interdependence and inflammatory pathways that potentially affect the entire circulation. Although treatments specific for COPD-related cardiovascular and pulmonary vascular disease are limited, early diagnosis, study of pathophysiology, and monitoring the effects of treatment are enhanced with improved imaging techniques. In this article, we review recent advancements in the imaging of the vasculature and the heart in patients with COPD. We also explore the potential mechanism of coupling between the progression of COPD and vascular disease. Imaging methods reviewed include specific implementations of computed tomography, magnetic resonance imaging, dual-energy computed tomography, positron emission tomography, and echocardiography. Specific applications to the proximal and distal pulmonary vasculature, as well as to the heart and systemic circulation, are also discussed.
Figures
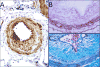


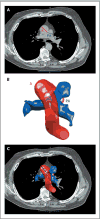




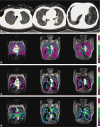
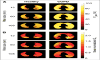
References
-
- Divo M, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61. - PubMed
-
- Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):39S–51S. - PubMed
-
- Santos S, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

