A cool hybrid approach to the herpesvirus 'life' cycle
- PMID: 24553093
- PMCID: PMC4031633
- DOI: 10.1016/j.coviro.2014.01.008
A cool hybrid approach to the herpesvirus 'life' cycle
Abstract
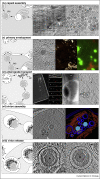
Electron cryo tomography (cryoET) is an ideal technique to study virus-host interactions at molecular resolution. Imaging of biological specimens in a frozen-hydrated state assures a close to native environment. Various virus-host cell interactions have been analysed in this way, with the herpesvirus 'life' cycle being the most comprehensively studied. The data obtained were further integrated with fluorescence and soft X-ray cryo microscopy data applied on experimental systems covering a wide range of biological complexity. This hybrid approach combines dynamic with static imaging and spans a resolution range from micrometres to angstroms. Along selected aspects of the herpesvirus replication cycle, we describe dedicated combinations of approaches and how subsequent data integration enables insights towards a functional understanding of the underlying processes.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

