The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells
- PMID: 24553136
- PMCID: PMC6258903
- DOI: 10.1038/nature12998
The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells
Abstract
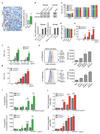
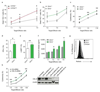
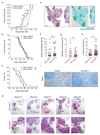
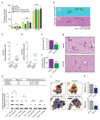
Tumour metastasis is the primary cause of mortality in cancer patients and remains the key challenge for cancer therapy. New therapeutic approaches to block inhibitory pathways of the immune system have renewed hopes for the utility of such therapies. Here we show that genetic deletion of the E3 ubiquitin ligase Cbl-b (casitas B-lineage lymphoma-b) or targeted inactivation of its E3 ligase activity licenses natural killer (NK) cells to spontaneously reject metastatic tumours. The TAM tyrosine kinase receptors Tyro3, Axl and Mer (also known as Mertk) were identified as ubiquitylation substrates for Cbl-b. Treatment of wild-type NK cells with a newly developed small molecule TAM kinase inhibitor conferred therapeutic potential, efficiently enhancing anti-metastatic NK cell activity in vivo. Oral or intraperitoneal administration using this TAM inhibitor markedly reduced murine mammary cancer and melanoma metastases dependent on NK cells. We further report that the anticoagulant warfarin exerts anti-metastatic activity in mice via Cbl-b/TAM receptors in NK cells, providing a molecular explanation for a 50-year-old puzzle in cancer biology. This novel TAM/Cbl-b inhibitory pathway shows that it might be possible to develop a 'pill' that awakens the innate immune system to kill cancer metastases.
Conflict of interest statement
The authors declare competing financial interests: the Lead Discovery Center GmbH (Dortmund) and Axel Ullrich (Munich) have patented the compound used in our study; Josef M. Penninger holds shares in a company that attempts to develop Cbl-b modulators.
Figures










Comment in
-
Immunotherapy: killing with natural killers, naturally.Nat Rev Clin Oncol. 2014 Apr;11(4):180. doi: 10.1038/nrclinonc.2014.39. Epub 2014 Mar 4. Nat Rev Clin Oncol. 2014. PMID: 24590132 No abstract available.
-
Signalling: loss of Cbl-b unleashes anti-metastatic natural killer cells.Nat Rev Cancer. 2014 Apr;14(4):218. doi: 10.1038/nrc3707. Epub 2014 Mar 13. Nat Rev Cancer. 2014. PMID: 24622519 No abstract available.
-
Cancer: Unleashing NK cell anti-metastatic activity.Nat Rev Drug Discov. 2014 Apr;13(4):257. doi: 10.1038/nrd4297. Nat Rev Drug Discov. 2014. PMID: 24687061 No abstract available.
References
-
- Lacour F, Oberling C, Guerin M. Effect of dicoumarol on the development of metastases of the T8 epithelioma in the rat; new research. Bull Assoc Fr Etud Cancer. 1957;44:88–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

