C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma
- PMID: 24553141
- PMCID: PMC4050669
- DOI: 10.1038/nature13109
C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma
Erratum in
- Nature. 2014 Apr 24;508(7497):554. Becksford, Jared [corrected to Becksfort, Jared]
Abstract
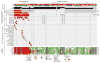
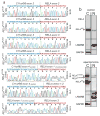
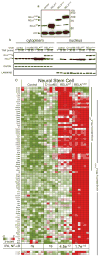
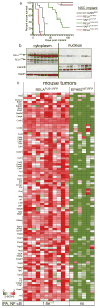
Members of the nuclear factor-κB (NF-κB) family of transcriptional regulators are central mediators of the cellular inflammatory response. Although constitutive NF-κB signalling is present in most human tumours, mutations in pathway members are rare, complicating efforts to understand and block aberrant NF-κB activity in cancer. Here we show that more than two-thirds of supratentorial ependymomas contain oncogenic fusions between RELA, the principal effector of canonical NF-κB signalling, and an uncharacterized gene, C11orf95. In each case, C11orf95-RELA fusions resulted from chromothripsis involving chromosome 11q13.1. C11orf95-RELA fusion proteins translocated spontaneously to the nucleus to activate NF-κB target genes, and rapidly transformed neural stem cells--the cell of origin of ependymoma--to form these tumours in mice. Our data identify a highly recurrent genetic alteration of RELA in human cancer, and the C11orf95-RELA fusion protein as a potential therapeutic target in supratentorial ependymoma.
Conflict of interest statement
None.
Figures
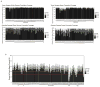

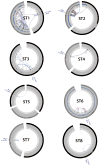
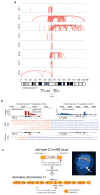
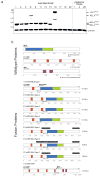

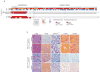
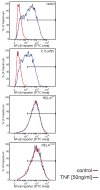
Comment in
-
Cancer: Tumours outside the mutation box.Nature. 2014 Feb 27;506(7489):438-9. doi: 10.1038/nature13061. Epub 2014 Feb 19. Nature. 2014. PMID: 24553138 No abstract available.
References
-
- Kleihues P, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. discussion 226–219. - PubMed
-
- Modena P, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–5233. - PubMed
-
- Taylor MD, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. - PubMed
-
- Puget S, et al. Candidate genes on chromosome 9q33–34 involved in the progression of childhood ependymomas. J Clin Oncol. 2009;27:1884–1892. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

